With the availability of a commercial instrument for the streaming potential measurement in the 1990s, the surface zeta potential has started to become an indispensable parameter for the characterisation of polymer membranes.
Measuring the streaming potential requires the formation of a capillary flow channel by the membrane sample. A pressure is then applied on an aqueous solution of known composition and the flow of liquid through the capillary channel provokes a DC electric potential, which is the streaming potential. This is related to the surface zeta potential.
Different sample configurations are applicable depending on the shape of the membrane and the location of the active surface. Flat sheet membranes are preferably mounted in a parallel-plate arrangement to create a single-flow channel with a rectangular cross-section and a typical sample size of 20 mm x 10 mm. Alternatively, membranes for microfiltration (MF) and ultrafiltration (UF) offer the ability to conduct a zeta potential analysis inside pores by applying a pressure difference across the corresponding membrane sample.
Hollow fibre membranes are prepared as a fibre bundle embedded in a tubular support to characterise the zeta potential at the inner surface. Similarly, capillary membranes are mounted in a sample holder with an appropriate mechanism to adjust the inner diameter in accordance with the requirement of the streaming potential measurement.
The zeta potential is responsible for the electrostatic interaction between the membrane surface and solutes in the feed. Furthermore, the zeta potential is used as an indicator for membrane surface charge, which is otherwise difficult or even impossible to determine.
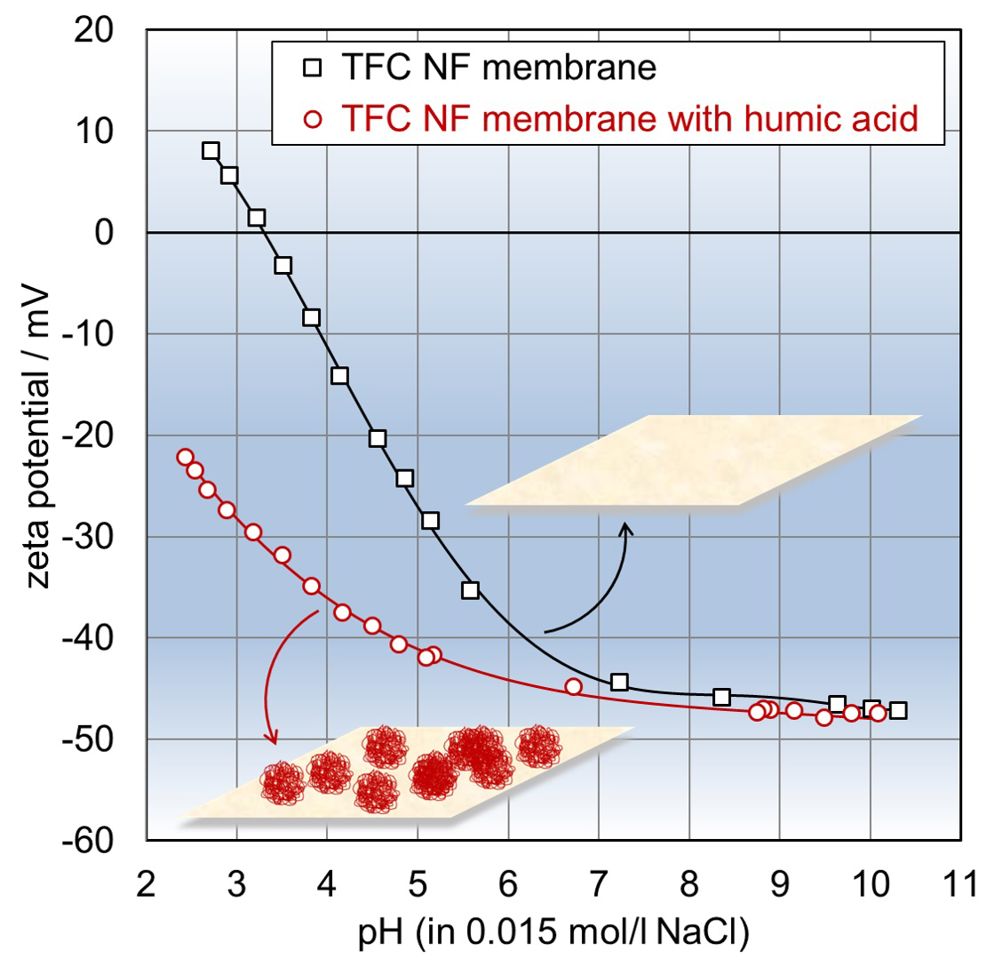
Membrane surface charge dominates the rejection of solutes, especially in UF and nanofiltration (NF) processes. Most polymer membranes exhibit a negative charge in a wide range of feed water pH and thus preferably repel anionic compounds and divalent anions. The membrane charge can be tuned with an appropriate surface modification to favour the rejection of specific solutes.
Membrane fouling
Polymer membranes are prone to membrane fouling. Depending on the application, membrane performance may decline by organic fouling, scaling, biofilm formation, or a combination of these fouling processes. Again, the membrane charge and the surface zeta potential contribute to the fouling propensity of polymer membranes. Oppositely charged foulants in the feed solution get attracted towards the membrane surface, thereby increasing the probability of membrane fouling.
Alike charges of the membrane surface and foulants in the feed provoke electrostatic repulsion and help to suppress membrane fouling. The sensitivity of the zeta potential to the outermost membrane surface and the separation of surface and interfacial phenomena from the bulk properties of the feed make the streaming potential method also suitable for the characterisation of the dynamics of solute-on-membrane surface adsorption processes.
In the past 25 years, the diversity of information provided by the zeta potential has been exploited to better understand membrane performance. The different attempts to apply the surface zeta potential involve:
- studying the effect of solution chemistry;
- modelling salt retention;
- determining the initial rate of membrane fouling;
- qualifying membrane surface modification;
- evaluating the efficiency of cleaning cycles;
- demonstrating the attack of harsh cleaning chemicals; or
- illustrating adsorption processes of different solutes on the membrane surface.
Author
Thomas Luxbacher, principal scientist at Anton Paar GmbH.






