Hydrogen is inherently a green fuel. It produces no toxic emissions, the only by-product of its combustion being water vapor. As a result, it is being heralded as the clean fuel of the future and is set to play a key role in the transition from fossil fuels to renewable energy. However, most of the hydrogen produced today is sourced from the coal or natural gas industry, from steam reforming natural gas. The process to make this so-called ‘grey hydrogen’ requires vast amounts of energy while emitting significant amounts of carbon dioxide into the atmosphere. While less damaging than coal-fired power, for example, this still does not provide a solution to the green energy challenge.
A more sustainable solution is the use of carbon capture technologies to trap CO2 before it escapes into the atmosphere. Termed ‘blue hydrogen’, this tackles the pressing emissions issue, however the captured CO2 still needs to be stored, which comes with its own challenges when deploying at scale.
The panacea that the industry is trying to reach is the production of ‘green hydrogen’ – hydrogen produced by the electrolysis of water and powered by renewable energy sources, like wind or solar. Creating green hydrogen has been proven technically possible, but scaling up has proved extremely difficult. It is filtration and separation solutions that may provide the key to unlocking the green hydrogen challenge and unleashing the potential of this potentially transformational energy source.
Scaling up
A combination of technological constraints, regulatory challenges and cost issues have made green hydrogen thus far impossible to produce at industrialized levels – and in each of these three areas, filtration technologies could hold the key.
The electrolysis that is the basis of the manufacturing process involves the dissociation of the water molecule in an electric field. Hydrogen is then produced at the cathode and oxygen at the anode with an electrolyte present in between the electrodes.
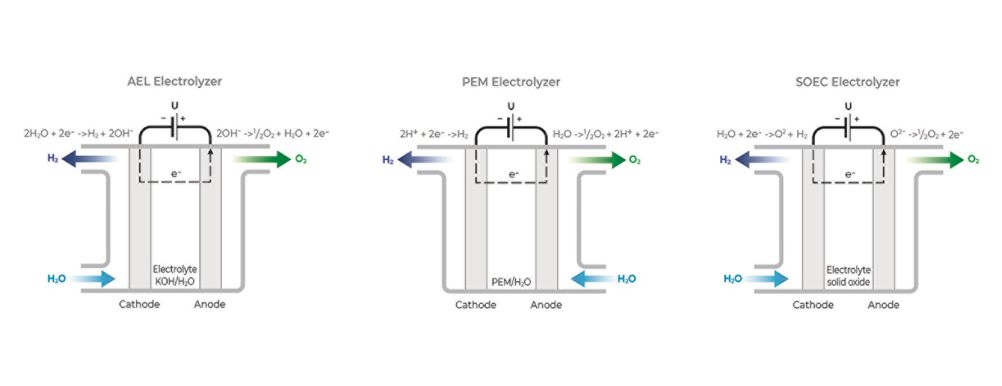
There are three types of electrolyzers used to achieve this. The alkaline electrolyzer (AEL) uses liquid potassium hydroxide as the electrolyte and is the most widely used in industrial applications, but comes with some drawbacks, including lower purity levels and lower energy efficiency. Polymer electrolyte membrane (PEM) uses a solid polymer electrolyte and is increasingly being favoured because it has fewer of these drawbacks but is still expensive compared to AEL. Finally, solid oxide electrolyzers (SOEC) use a solid ion-conducting ceramic electrolyte – a technology that holds great potential but is still in early stages of commercialization.
Regardless of the electrolysis technology used, the hydrogen stream produced needs to be further processed to remove solid, liquid and gaseous contaminants. It is in this process that the real technical challenge lies because the regulations around gas purity specifications are extremely stringent. You would typically see concentrations between 2,000-6,000 ppm of oxygen and over 2,000 ppm of water in hydrogen using commercial alkaline electrolysis. The maximum concentration allowed for fuel cell vehicles is 5 ppm of each.
The balancing act
AEL systems – the most widely used in industrial applications – require further optimization to produce green hydrogen at a large scale and plug the gap between outputs and purity standards. Several unit operations using filtration, separation and purification technology are needed to achieve the required purity levels.
Once the hydrolysis has taken place, the liquid/gas mixture needs to be cooled, separated, and compressed. However, cooling the gas has major cost implications and can be as high as 5% of the total system outlay. Gravity separators, mist eliminators pads, filter vane separators and more recently liquid/gas coalescers are used to separate the liquid contaminants. These typically require large housings and must be operated at low velocities to prevent liquid re-entrainment.
To add to the purification costs, solid contaminants, originating from oxidation in process piping and equipment such as pumps and compressors, must be eliminated. Adsorbent fines, if they are being used, situated in the final drying equipment may also get released, contaminating the gas. To remove the solid contaminants, regenerable and disposable gas filters in different micron ratings are deployed throughout the process.
The final step is the efficient storage of the hydrogen once it is produced. It can be compressed and stored in tanks, pumped into salt caverns, or converted into liquid ammonia, using Haber-Bosch Synthesis. Optimization of all these processes is required to industrialise the production of green hydrogen at a scale where it becomes a viable replacement for fossil fuels.
Solving the equation
There is no doubt that hydrogen will play a role in our clean energy future, but creating it in a truly green way requires the use of electrolyzers powered by a renewable energy source, such as solar or wind, and filtration and separation technologies will have a huge part to play in making these renewable-powered electrolyzers commercially viable.
New breakthroughs are made possible every day. We are now seeing more and more companies looking to partner with Pall Corporation for R&D and strategic support, as well as products – working together to implement new innovations and find solutions to these complex challenges.
Government regulation also plays an important role. The Green Deal in Europe and a range of tax incentives in the USA are encouraging innovation in this space and allowing companies to invest and move forward. If renewable energy companies and their supply chain seize this opportunity and continue to work together to develop solutions for this rapidly evolving market, we will be able to convert these ideas and innovations into reality.
Ultimately this is an equation that we need to solve. The impact of continuing down the path we are on is being revealed before our very eyes in the form of wildfires, flash floods, hurricanes, and more. We owe it to ourselves and to future generations to continue to innovate until we can find a solution, whether that is in the form of green hydrogen or another sustainable power source – and it’s inspiring to know that filtration and separation technologies will have such a vital role to play in tackling this pressing global challenge.
Author
Dr. Maria Anez-Lingerfelt, Senior Scientist in Pall Corporation’s Application Development Team.