
It could be used to clean up spills or in routine drilling, such as in the deep ocean as well as on land, where water is injected into wells to help force oil out of deep rock formations. In this case the mixed oil and water that’s extracted is put in large tanks to allow separation by gravity; the oil gradually floats to the top, where it can be skimmed off.
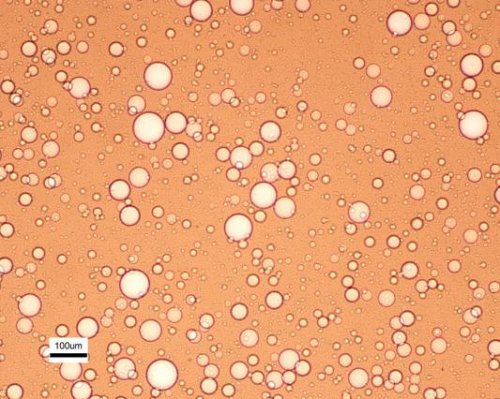
That works well when the oil and water are already partly separated, but difficulties arrive when they have emulsion, with very tiny droplets of oil stabilized in a water background, or water in an oil background. The difficulty significantly increases for nanoemulsions, where the drop sizes are below a micron.
To break down those emulsions, crews use de-emulsifiers, which can themselves be environmentally damaging. In the 2010 Deepwater Horizon oil spill in the Gulf of Mexico, for example, large amounts of dispersants and de-emulsifiers were dumped into the sea.
Pore structures
In the case of land-based drilling, where so-called “produced water” from wells contains fine emulsions of oil, companies sometimes simply dilute the water until it meets regulatory standards for being discharged into waterways.
The new approach developed by the researchers, led by MIT professor Kripa Varanasi, uses membranes with hierarchical pore structures. The membranes combine a very thin layer of nanopores with a thicker layer of micropores to limit the passage of unwanted material while providing strength sufficient to withstand high pressure and throughput. The membranes can be made with contrasting wetting properties so their pores either attract oil and repel water, or vice versa.
“This allows one material to pass while blocking the other with little flow resistance,” said Varanasi. The choice of membrane, or a combination of both, could be based on which material predominates in a particular situation, he explains.
Small-pore membranes
The pores have to be smaller than the droplets in order to block them, Varanasi says — which, in the case of nanoemulsions, leads to very small pores and significant flow resistance, limiting the throughput. Throughput can be improved by increasing the pressure gradient or making the separation layer very thin, but past attempts to make such thin, small-pore membranes have yielded materials that tear even under nominal pressure. The team’s solution was to make large holes on one side that penetrate most of the way through the material, providing little resistance to flow, and nanoscale holes on the other surface, in contact with the emulsion to be separated. The thin layer with nanoscale pores allows for separation, and the thick layer with large pores provides mechanical support.
They make the membrane by pouring a polymer solution onto a glass plate. This casting plate is then immersed in a nonsolvent bath to induce precipitation to form a film. The technique creates a bilayered polymer phase: one layer is polymer-rich, and one is not. As they precipitate out, the polymer-rich phase develops the smaller pores; the polymer-lean phase makes the larger ones. Since the solutions form a single sheet of film, there is no need for bonding layers together, which can result in a weaker filter. As a final stage, a different polymer is added to give the material — including the lining of the pores — surfaces that attract or repel oil and water. The skin layer thickness can be further optimized using polymeric pore formers to enhance throughput.
The team says that the membrane could be manufactured at industrial scale, to process large quantities of the finely mixed materials back into pure oil and water. The membranes could be made using a high-volume, roll-to-roll manufacturing system, so it should be relatively easy to achieve large-scale production, according to Varanasi.
Increased separation
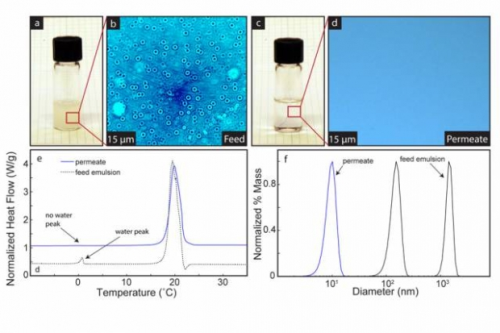
The team tested the membranes in separating nanoemulsions while maintaining integrity at high pressure. The team used various techniques — including differential scanning calorimetry, dynamic light scattering, and microscopy — to test the separation efficiency, showing more than 99.9% separation.
Microscopy images show the membrane in operation, with dye added to the water to make the droplets more obvious. Within seconds, an oil-water mixture that is heavily clouded becomes perfectly clear, as the water passes through the membrane, leaving pure oil behind.
The process is described in the journal Scientific Reports by MIT professor Kripa Varanasi, graduate student Brian Solomon, and postdoc M. Nasim Hyder. The team is working with Shell, which supported the research through the MIT Energy Initiative, to further test the material.





