
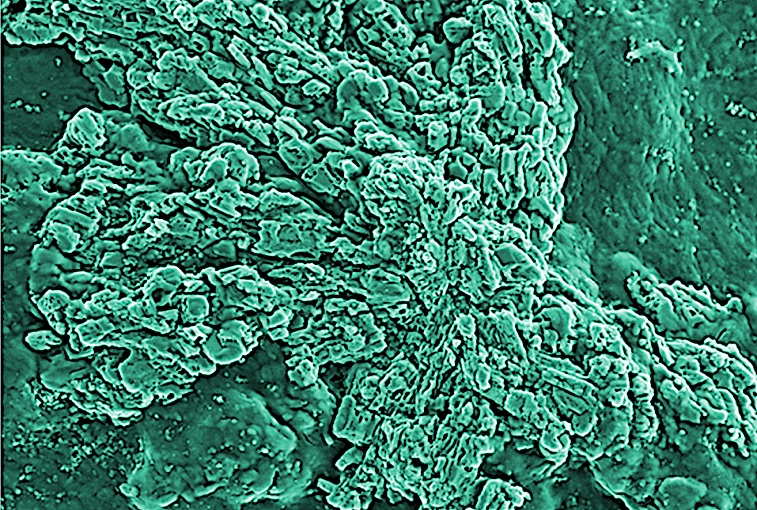

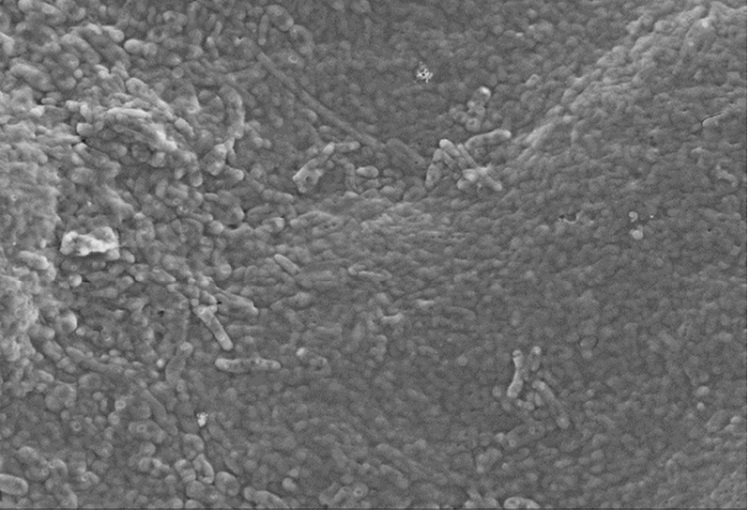
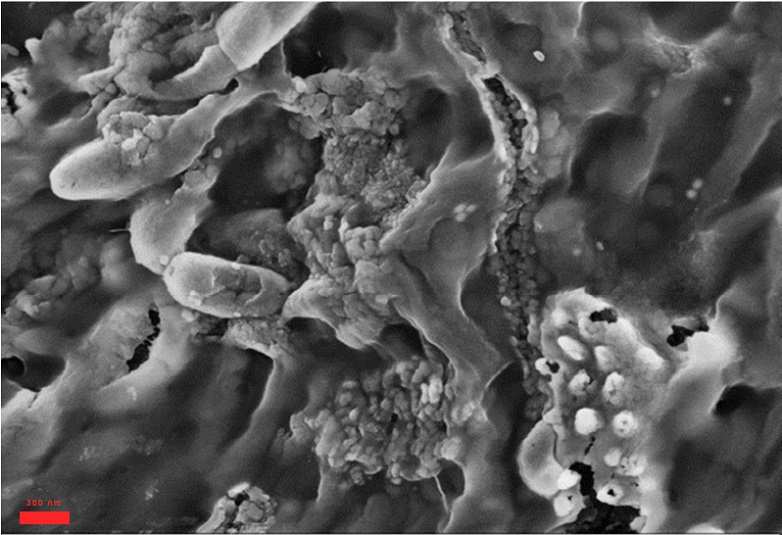
Biofouling in water reverse osmosis remains a severe and recurrent problem. The reasons are complex, but biochemistry has made great strides over the last 20 years and new discoveries can help to find the best approach to the problem.
After several decades of trial and error, "why” seems to be the right question when it comes to biofouling in water reverse osmosis. The issue continues to reoccur and disinfectants like sodium hypochlorite, which are currently widely used, sometimes fail to mitigate bio-contamination or the bio-fouling issue. Bio-fouling problems Each bio-fouling case should be cautiously considered as unique. In the past, a lack of solid, practical knowledge led to the emergence of certain accepted practices which spread and further contributed to confusion about the best methods to adopt. As a result, biofouling and its control have remained a major operating problem for most reverse osmosis (RO) plants, particularly those in tropical and sub-tropical regions.
"Bacteria can share some genetic material blocks and can share their 'past living-experience'.....capable of messaging complex information to distant targets and across generations."
In the late 1990s, two strategies were strongly recommended for the prevention and control of membrane biofouling. These were the physical removal of bacteria from the feedwater using micro or ultrafiltration pre-treatment and an aggressive metabolic chemical inactivation of bacteria through the use of biocide and UV radiation. Historically, treating biofouling in a membrane water treatment system was based on that dichotomy – the extracting versus killing approach, which essentially ignored some of the specific long-term adaptive skills and capacity of the bacteria, such as its genetic rapid adaptation to unfavourable conditions.
Bacteria basics Before attempting a description of the “why?” question, it is important to recall some basic general features of bacteria: • Biological life is present everywhere, on any surface including polished stainless steel. Bacteria were the first inhabitants on earth and successfully adapted to various harsh conditions. • Living bacteria are adaptive and thus sometimes considered as “smart cells”, because of their ability to react to stress situations. Scientists established that bacteria can share some genetic material blocks, like RNA, and thus can share their “past living-experience”. RNA is a likely mediator of some adaptation because it is a mobile molecule capable of messaging complex information to distant targets and across generations1. • Microorganisms can build thick defence walls when exposed to a changing environment which triggers a stress response. These bio-polymeric walls act as a physical and chemical protection barrier. • Microorganisms need food assimilable organic carbon (AOC) or biodegradable dissolved organic carbon (BDOC), water and some mineral elements like the phosphate anion that ensures the capacity to replicate genetic materials during the growth and expansion phase of a bacteria colony2. • Those four basic characteristics of bacteria highlight how challenging it is to keep a bio-contamination or a bio-adaptation under control. At this stage, we may already question the relevance of past standard practices which damage the EPS walls during a Cleaning in Place (CIP) protocol. Indeed, was the mechanically triggered diffusion of messaging RNA the root of a faster adaptation evolution to biocidal treatments, making some bacteria super-resistant even to chlorine treatment?
"Historically, treating biofouling in a membrane water treatment system ...essentially ignored some of the specific long-term adaptive skills and capacity of the bacteria, such as its genetic rapid adaptation to unfavourable conditions."
Understanding strategies The five characteristics below, based on the latest scientific discoveries, will hopefully shed some light on the strategies used by bacteria to protect themselves. This may help to gradually adopt the right approach to attain a bio-controlled RO plant, moving on from the recurrent failures of the past to a more successful future.
1) Disinfectant diffusion in protective biofilms The multiple layers of bacteria cells and the bio-polymeric material released (EPS) constitute a compact structure within which biocides find it difficult to penetrate. For example, the chlorine levels measured within biofilms of P. aeruginosa and K. pneumoniae only reached 20% of the concentrations measured in the bulk liquid3.
In addition, the diffusion inside the bio-polymer layer is strongly reduced – data shows it is reduced by a factor of 60x. Similarly, the penetration of biocide to the centre of an S. epidermidis biofilm cluster took 60 times longer than the time estimated for a free diffusive access.
2) Phenotypic adaptations of biofilm cells to sub-lethal concentrations of disinfectant We know from antibiotic medical drug science that dosing low amounts of “killing” substances can give rise to adaptation and resistance of bacteria to that substance. This is now a well-known process. Surviving bacteria, which experienced a specific form of stress, have activated certain defensive/adaptation genes (RNA will message the evolution) and before dying, can release their genetic messengers’ material (which has registered their "life experience"), making it available for another cell.
Dr. Andrea Calixto advocates the idea that the export, travel and import of small RNAs between bacteria and host is an active and targeted process and there is increasing evidence pointing in that direction. In other words, the cells that survived a disinfection attack could possibly pass on the genetic response increasing the resilience of the next generation of cells. As a result, it is now well established that, for example, an intensive chlorine shock dosing is preferable to a daily small continuous dosing.
3) Phenotypic adaptation of cells in a biofilm environment Before cells become mature, they sense their environment in different ways. This sensing helps bacteria to activate or deactivate some genetic material to better adapt and resist to a new or specific environment. Past studies have shown for stressed bacteria that genes coding flagellar proteins are repressed and genes coding for EPS and adhesin proteins are induced4.
This defence strategy is based on improving cell adhesion to a surface to build a thick layer and walls where bacteria will mitigate their stress. These changes induced by cell adhesion can lead to the appearance of more resistant phenotypes, as suggested by studies reporting the greater resistance of cells that are merely adhered to a surface when compared with their planktonic counterparts.
4) Pathogen community protection in multispecies biofilms Some bacteria can protect others, which was quite unknown until recently. Cell-to-cell communication has been identified as controlling biofilm development in many bacterial species. In complex consortia, species’ interactions can lead to the emergence of specific biofilm phenotypes. Consistent with these findings, regulation of the stress response by quorum sensing has recently been reported in other species.
A recent study reported that the food pathogen E. coli O157:H7 formed a biofilm with a 400-fold higher volume when it was grown in association with Acinetobacter calcoaceticus, a meat factory commensal bacterium, rather than in a monoculture. The details of this cooperative mechanism remain unclear. Because the specific nature and composition of a multi-species biofilm matrixis critical, every water treatment plant must be considered unique in terms of its bacteriology approach and anti-biofouling strategy. 5) The Persisters’ Type This last characteristic behaviour is rather unknown in the field of water treatment. Scientists discovered recently that some type of bacteria inside a biofilm could enter a protective mode and survive harsh conditions – these were named the persisters. An illustration of the adaptation of specific phenotypes that may contribute to the bacterial resistance observed in biofilms is that a small fraction of the population may enter a highly protected state displaying dramatic resistance. In 2018, a biologist discovered that some bacteria can survive aggressive conditions by pausing their metabolic activity. Analysis revealed that genes involved in guanosine tetraphosphate synthesis are upregulated in persisters, which represses transcription and DNA replication and leads, for example, to antibiotic tolerance. These cells are phenotypic variants but not genetic mutants and have been identified in planktonic bacterial populations. One assumption is that persisters develop more frequently in a biofilm than in a planktonic culture, perhaps induced by the specific environmental conditions prevailing within the structure and may therefore contribute to better antimicrobial protection in the biofilm. These five characteristics highlight how complex and challenging it is to anticipate a bacterial behaviour. In addition to food scarcity or availability, the latest discoveries demonstrate that specific behaviours can take place where bacteria will find a way to circumvent an aggressive killing approach. The diffusion/reaction limitation within a biofilm structure is one of the main mechanisms implicated in bacteria resistance to disinfectants. That defence mechanism is the result of a past learning or adaptation which was chemically recorded in a bacteria's genetic material and later on transferred to a +1 generations via RNA messengers. Finding solutions It is no surprise then to see that the negatively charged EPS polymer will block the diffusion of hypochlorite anions (electrostatic repulsion of negatively charged molecules). One solution in that case is to reduce the pH of hypochlorite solution to allow diffusion of the neutral hypochloric acid molecule. Bacteria do not yet have any defence against the oxidation power of HOCI. At the same time, a mechanical breakdown of the EPS matrix runs the risk of RNA messaging leakage, in addition to food dissemination. In such a case, a complete oxidation process via the use of chemical substances or UV light will be necessary, but may not be enough, as even halogenated organic molecules could serve a food for some bacteria. To face the adaptive evolution of bacteria, scientists recommend using a multi-parameter approach or synergies in disinfectant. In this respect, synergistic actions between two or more processes/molecules have been reported in numerous peer reviews. The effect observed was stronger than might have been predicted. For example, the combinations of sodium hypochlorite and hydrogen peroxide, or Cu2+ ions and quaternary ammonium, or eucalyptus oil and chlorhexidine, or silver and surfactant, or bacteriophage and alkaline cleaner – there are many synergistic combinations. Bacteria needs to be killed multilaterally to ensure the avoidance of any resistance which could cause problems later. Exotic physical treatments reported include the use of low-intensity ultrasonic agitation combined with chlorhexidine to rapidly damage bacteria nuclei and envelopes, or the use of ultraviolet light combined with chlorine dioxide. However, until such techniques are established on an industrial scale and in a manner not detrimental to the polymer membranes used in today’s desalination technology, the holy grail in the field of daily RO operation remains to keep the food availability (AOC) minimal and under control. The amount of foodstuff in system feed ultimately controls the population of microorganisms and any resulting biofouling.



