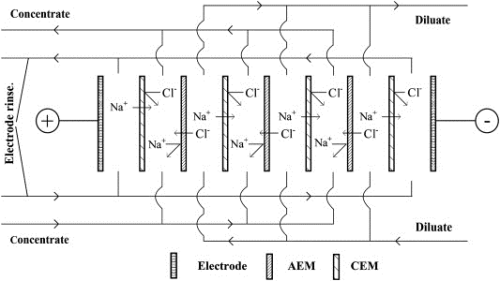
This review by Polish researchers – in a Special Issue of Desalination on Removal of Boron from Seawater, Geothermal Water and Wastewater – summarises and discusses reports in the literature involving boron transport and removal using ion-exchange membranes.
Most of these previous reports concern boric acid or borate transport in electrodialysis (ED) systems. The reported rates of boric acid transport in ED are so small that it has been suggested that ED may be effective in separating strong mineral acids and ions from boric acid in a simple electrodialytic desalination process.
This appears to be the most promising application of ion-exchange membranes for the treatment of boron-containing waters.
Although the reported rates for borate transport in ED are high, the electric current efficiencies for borate transport are low because of low borate mobility. Moreover, borates exist only at high pH, at which there is a serious risk of membrane scaling with basic compounds of elements such as calcium and magnesium.
Therefore, the diluate solution must be demineralised before borate removal by ED, which challenges the economic feasibility of the entire process.
Tentative reports on boron removal by Donnan dialysis (DD) have not provided much information about the effectiveness of boron removal from waters or its final concentration.


