Halo-benzoquinones (HBQs) are a recently identified group of disinfection by-products, which have been observed to be present in chlorinated drinking waters.
Here Canadian researchers investigate the link between natural organic matter (NOM) characteristics and HBQ formation during bench-scale coagulation of raw water.
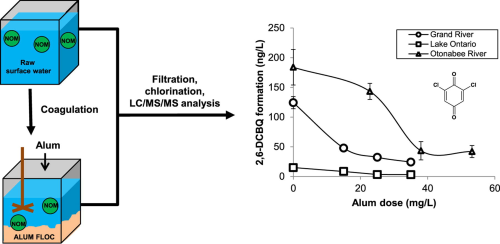
Three source waters (from Lake Ontario, Otonabee River, and Grand River) were subjected to jar testing using alum followed by chlorination. NOM fractions were analysed via liquid chromatography–organic carbon detection (LC–OCD), while HBQs were quantified using liquid chromatography–triple quadrupole mass spectrometry.
One HBQ, 2,6-dichloro-(1,4)benzoquinone (2,6-DCBQ), was identified in all waters after chlorination, and appeared to decrease with increased applied alum dose. The 2,6-DCBQ exhibited high correlations with some humic NOM indicators: humic substance concentration (in Grand and Otonabee River waters only), UV absorbance at 254 nm, UV absorbance at 254 nm of the humic peak, and specific UV absorbance of humics (humic SUVA).
With data pooled from the three waters, the biopolymer fraction of NOM was most strongly correlated with 2,6-DCBQ formation (R2 = 0.78, p < 0.001). This may be due to co-removal of biopolymers with HBQ precursors during coagulation.
These results indicate that coagulation processes can be effective for reduction, but not elimination, of HBQ precursors.
Water Research, Volume 47, Issue 5, 1 April 2013, Pages 1773–1782.



