Phosphate is widely used in agriculture and other industries, but its extensive use yields large amounts of phosphate-containing wastewater. This can be released from many point and non-point sources such as agricultural fertiliser runoff, treated and untreated municipal wastewater, and effluents from the chemical and food processing industries.
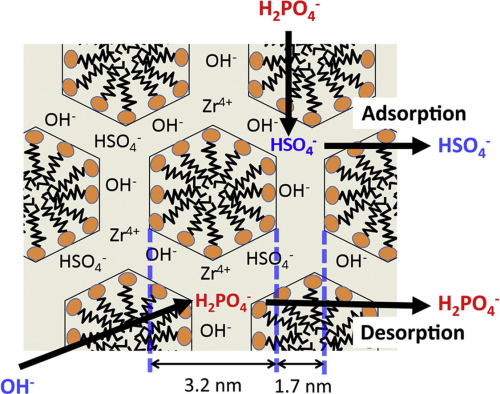
Researchers at Hokkaido University in Japan have now synthesised a zirconium sulfate-surfactant micelle mesostructure (ZS), and investigated its capacity for phosphate removal from water.
The material’s phosphate adsorption kinetics, the effect of pH and interfering anions, adsorption isotherm, desorption capacity, and reusability have been investigated.
The ZS was an effective adsorbent for phosphate with a very high adsorption capacity. The phosphate adsorption capacity increased with decreasing pH.
Although the adsorption of nitrate, chloride and acetate ions was negligible, bicarbonate ions were found to be possible interfering anions.
The adsorbed phosphate was desorbed effectively using NaOH solution. Since breakage of ZS particles resulted when using NaOH, ZS was immobilised on a polymer matrix, and a 50-cycle adsorption–desorption test was carried out to determine the ZS-immobilised polymer (P-ZS) reusability.
The P-ZS retained its functionality and adsorption and desorption capacity over 50 cycles without loss of original capacity.
A phosphate solution containing about 10 mg P/L was treated in a column packed with P-ZS. The phosphate could be adsorbed completely onto P-ZS up to 1020 bed volumes.
These results clearly indicate that ZS is a highly effective adsorbent for phosphate, and enables the removal of phosphate from water.
Water Research, Volume 47, Issue 11, 1 July 2013, Pages 3583–3590.


