

Manufacturing biotechnology drugs is one of the driving forces in the U.S. economy. Strong growth is projected for biotechnology drug sales, and the demand for monoclonal antibodies (mAbs) is expected to grow at a rate of 15% through 2014, from an estimated 8,000 kilograms (kg) in production in 2009. Other biotechnology products, including vaccines, are also expected to grow, by at least 9% in the same period [1].
Produced at the industrial scale using various recombinant cell lines, biopharmaceutical products must have very high purity, with the concentration of host cell proteins and DNA reduced to the range of parts per million relative to the desired product, or lower. The final product must also be sterile (no viable micro-organisms present), and should contain less than 10 nanograms of NDA per dose and less than one virus per million doses [2].
The cost of manufacturing products to this standard is very high, and the expansion of demand has come hand in hand with pressure to increase efficiency, reduce production costs, and speed time to market of products. At the same time, there has been an increase in concern about the susceptibility of a phase 1 drug to contamination or cross-contamination with other substances being produced in a research facility.
These concerns are affecting the methods used to produce therapeutic mAbs and other therapeutic proteins used in the making of biotechnology products. One of the key trends resulting from these pressures has been the huge growth in the adoption of single-use technologies. Single-use solutions increase product throughput, minimise non-revenue generating cleaning (and its associated cleaning validation steps), reduce or eliminate cross contamination, and ultimately make it possible to develop lower cost facilities.
Single-use solutions complement the increasing prevalence of higher titer, smaller batch processes that reduce product costs, allowing for greater process flexibility. Single-use solutions also have the potential for operation in the uncontrolled unclassified ‘grey space’, outside the costly clean room.
Existing protein purification technology
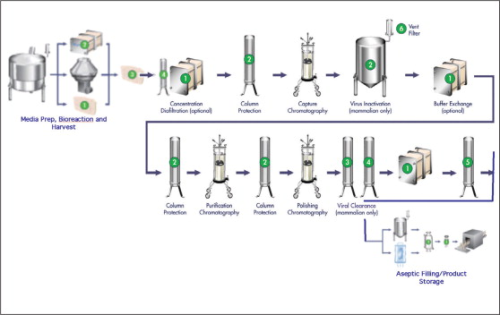
The stringent purification of mAbs has traditionally been achieved by a protein purification process similar to that shown in Figure 1. The purification process begins with media preparation, bioreaction and harvest, and then moves into concentration and diafiltration. From there the mAb or other protein product is purified during several polishing and chromatography steps along with a virus clearance/filtration step. The mAb is then moved into a final formulation step, followed by sterile filtration and aseptic filling and product storage.
The bulk of the work has traditionally taken place inside stainless steel equipment, hard-wired with dedicated piping, located in clean rooms with controlled, low levels of environmental pollutants such as dust, airborne microbes, aerosol particles and chemical vapours. Such clean room environments are costly to build and maintain.
As the field of biopharmaceuticals has evolved, it has become clear that biopharmaceutical manufacturers required the ability to quickly change over a facility to adapt to and manage multiple batch sizes, volumes and products, sometimes executing one campaign one day, and a different one the next day. Stainless steel-based processes are difficult to adapt to the quick changeovers frequently part of today's drug exploration process. Typically, to change over to a different product, the entire system has to undergo a rigorous, expensive, and time-consuming cleaning process, which includes cleaning for every new batch of product produced, and cleaning validation for every new product processed.
These are some of the most time-consuming and least revenue-generating activities in the biopharmaceutical manufacturing process. In addition, the actual water and other resource costs associated with cleaning and waste treatment can be significant.
Single-use integrated with stainless steel technologies
Using single-use solutions in mAb processing is not a new idea; they have played a role in the traditional stainless steel-based technology for quite some time. Millipore introduced its original single-use solutions for clarification and media preparation more than 20 years ago. Typically, customers would buy the products, and then create their own processes based around the single-use solutions. They would work closely with Millipore to design custom single-use assemblies that were either integrated with typical stainless steel vessels, or developed as standalone single-use assemblies.
Customers could run a batch and then dispose of the assembly with no possible crossover with other products processed. Also, customers were able to achieve significant savings by reducing the time spent cleaning the current stainless systems, as well as the water and other resources used. These single-use solutions reduced cost, reducing time to market, and reduced risk, especially for products produced in batches of 1000 litres or less.
Mobius® FlexReady Solutions
After years of working with customers to adapt their stainless steel-based processing systems by developing custom assemblies that integrated single-use products, Millipore believed that it understood the difficulties associated with current technology and the challenges customers faced in bringing products to market.
Millipore has now combined industry knowledge of existing single-use components with knowledge of the process itself achieved from helping customers design systems using stainless steel equipment. The results are smaller, disposable processes that accommodate multiple drugs in one manufacturing suite, without encountering cross-contamination issues especially cited by FDA in multi-product facilities. This need is especially acute when drugs are in clinical trials, and companies need to make quick process switches to accommodate a variety of drugs in their portfolio.
To satisfy this underlying need, Millipore developed and recently introduced its Mobius® FlexReady Solutions (see Figure 2), which combine scalable single-use filters and assemblies with process-ready hardware platforms optimised for specific unit operations. Thus far, there are single-use Mobius FlexReady Solutions for clarification, virus filtration, tangential flow filtration (TFF), and media and buffer preparation. The units were designed for modularity, flexibility, and manufacturability, based on a detailed understanding of biopharmaceutical manufacturing constraints.
Significant engineering and device integration analysis went into the design of the hardware and the single-use flow path Flexware™ products that make up the Mobius FlexReady Solutions. The design ensures that the single-use flow path not only performs consistently in an end-user's process but is also manufactured consistently and robustly. Furthermore, each of the hardware and Flexware designs has been tested in the specific application to ensure that it meets the end-user requirements for a given process.
Each flow path has been precisely designed and tested to provide a consistent, standardised flow. All assemblies are gamma-irradiated for low bioburden operations and include everything needed in one package: all feed and drain lines, process containers, sterile connectors, and Millipore sterilising-grade filter assemblies, including engineering and validation support.
In addition to facilitating the quick changes needed by today's manufacturers, Mobius FlexReady Solutions help remedy space constraints. Costly to build and maintain, clean room space is at a premium and the rooms are often small and cramped. The Mobius FlexReady Solutions are designed to minimise the equipment's footprint in a clean room by incorporating interchangeable filter carts that can be used with all the systems.
Key features and benefits of the systems include:
• Modular, interlocking filter cart for use across all four systems with easy to interchange filter stands.
• Innovative developments:
– Filter support holders
– Unique mixing tank on TFF system
– Low dead volume t-connectors
• Easy and sterile disconnection using NovaSeal crimping tool.
• Gamma-irradiated single-use flow path with different size options.
While the ultimate goal is to develop a complete single-use process from beginning to end that can be operated in a grey space environment, Millipore has begun by targeting four key steps in the mAbs processing/purification train. The single-use solutions for these steps have been designed so the operator can remove the process container from one step and carry it directly over to the next step.
The Mobius FlexReady Solutions can process various batch sizes, enabling customers to scale up from process development to pilot scale. Since the Mobius FlexReady Solutions are designed with common materials, once the flow path has been validated there is no need to revalidate as batch sizes increase up to pilot scale. The hardware is designed such that some of the components within the smaller process development version can be carried over to the pilot scale, resulting in cost savings. For example, with the TFF unit, the filter cart can be re-used, and the customer would only have to buy a new pump cart and Pellicon filter holder.
Media filtration and buffers
Single-use solutions for buffer and media preparation maximise equipment utilisation and optimise capital expenditure, particularly in multi-product and multi-scale facilities. Millipore's Mobius FlexReady Solution for buffer/media preparation features disposable mixers between 100 L and 500 L. The solution incorporates pre-sized filters for typical cell culture media and buffer filtration applications for a range of volumes. The fully integrated system can be quickly changed to accommodate different process needs, including varying volumes and also features disposable, appropriately-sized product collection process containers and filter flush process containers.
Clarification
In the clarification step, single-use systems can adapt to changing surface area and media grade requirements as multiple products come through the pipeline and they eliminate the need for significant capital investment in a centrifuge. The Mobius FlexReady Solution for clarification uses Millipore's Millistak® depth filtration media for cell-culture clarification directly from a bioreactor. The system is designed to accommodate up to 5.5 m2 of depth filter media and sterilising filtration media downstream. Depending upon the cell density and viability, the system can be used to clarify bioreactors between 10 L and over 500 L.
Viral filtration and clearance
Fully integrated single-use systems for virus filtration are a relatively new addition to the disposable arena. The Mobius FlexReady Solution for Virus Filtration features Millipore's Viresolve® Pro parvovirus removal filters for rapid and efficient parvovirus clearance from protein solutions. They have been embraced by the industry due to assurances of high virus retention and fast and efficient processing. By using a pump-based filtration system rather than a pressure-vessel based system for constant-pressure operation, the Mobius FlexReady Solution for Virus Filtration offers maximum flexibility for use in a multi-scale and multi-product facility. The system can handle protein loads between 10 g and 1000 g depending upon filter area used and validated protein loading.
Concentration and Diafiltration
The Mobius FlexReady Solution for Tangential Flow Filtration (TFF) offers a fully disposable flow path, including retentate vessels with active mixing, and high performance Pellicon® 3 devices. The system features a novel single-use retentate process container with low point retentate return, vortex breaker and a levitating magnetic impeller — features that are typically found in stainless steel vessels. The use of an innovative low dead-volume tee connector enables users to employ traditional pressure transducers without the need for cleaning in place of disposable pressure transducers. The hardware and the disposable Flexware in this system are engineered to provide high protein recovery at high concentrations. The system offers improved economy and productivity due to simpler procedures, and low downtime between products/batches.
Aesthetics and ergonomics
Aesthetics was one area given much thought in the design process. The brightly coloured units reflect Millipore's desire to show in a vivid way how different this process is from the white and silver stainless steel that makes up the bulk of the typical equipment in a clean room.
The company chose to paint the stainless steel cabinets with the bright colours (which are nonetheless permanent and are compatible with clean room standards) because it did not want the new equipment to look like every other piece of stainless steel.
Engineering and device integration analysis focused on making sure that the equipment works as intended, down to the smallest detail. For example, consider tubing size, which has been designed to be just the right length for a clean room operator wearing double gloves. If tubing is shorter than a certain length, it would be difficult to handle. Too long, and it would be difficult to cut the tubing to precise lengths every time.
Space minimisation is another key issue. The Mobius FlexReady Solutions are designed to minimise the equipment's footprint in a clean room. Interchangeable filter carts can be used with all the systems. If a customer is running four operations, they do not need four skids in the clean room, but can use one cart with all the other pump carts. Figure 3 illustrates how the ergonomic design makes the systems easy to move where they are needed.
A further design development is the provision of a process container carrier tiltable to 90 degrees, making it easier for people of all heights to install process containers easily. Also, the feed pump actually sits at an angle so the process container can be drained completely, rather than taking a chance of leaving behind any of the very expensive protein.
Other single-use solutions available
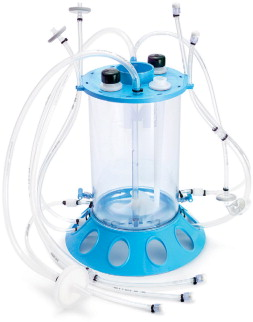
Most recently, Millipore introduced another single-use solution, the Mobius CellReady 3L Bioreactor, a ready-to-use three-litre bioreactor designed for bench-top, mammalian cell culture applications and in particular for process development laboratories (see Figure 4).
The Mobius CellReady 3L Bioreactor demonstrates all the benefits typically associated with single-use processing, while incorporating standard design features familiar to customers already using bench top, stirred-tank bioreactors. Designed to replace traditional glass bench top bioreactors, the Mobius CellReady 3L Bioreactor has a standard stirred-tank format for use in development and optimisation of cell culture processes. This vessel design provides a higher degree of predictability during process scale-up when compared to formats utilising alternative vessel designs and agitation methods. It features a universal design, ensuring compatibility with most bench top bioreactor control systems.
By eliminating time-consuming steps associated with cleaning, assembly, and sterilisation, the Mobius CellReady 3L Bioreactor significantly reduces the turnaround time typically associated with glass bioreactors. Whereas normal bioreactor turnaround time is between one to four days, with the Mobius CellReady 3L Bioreactor, it can be done in a matter of hours. This increases throughput, so more experiments can be done in the same laboratory than were historically possible.
Integration of systems with RFID technology
Using Radio Frequency Identification (RFID) technology to track products safely from the loading dock to the pharmacy has been the subject of much discussion. As RFID tags become integrated into such manufacturing components as filters, sampling process containers and biocontainers, they can alleviate burdensome manual data entry and more efficiently help the manufacturing process to conform to the standard operating procedures.
A manufacturer using RFID tagged filters can expect improvements in process efficiency by automating the exchange of data between the filter and batch record. Although similar improvements can be accomplished with barcodes, they can only be read line-of-sight and are susceptible to occlusion and inadvertent defacement. For example, at incoming inspection some manufacturers assign their own serial numbers to the filters and attach them as adhesive barcode labels. Within the manufacturing suite, the filters are electronically scanned into the batch record by the operator using a handheld barcode scanner. However, some manufacturers perform a preprocess integrity test and remove the outer packaging of the filters, thereby disconnecting the serial number from the filter. As there are now means to read the RFID tag within the metal housing, a filter with an integrated RFID tag can be identified in-situ during every process step [3].
RFID technology provides the opportunity to help customers carry such information as recipes, protocols, batch information, lot, and serial number on a tag. Since the RFID systems come with software and hardware, customers could write batch information on the tag. Millipore is investigating new ways to use RFID tags in the Mobius FlexReady Solutions. For example, a customer running a sequence from beginning to end could carry over batch record information from one process step to the next.
Sustainability issues a priority
Millipore is looking at sustainability issues with regard to its single-use Mobius FlexReady Solutions. Work continues on ways to recycle the single-use products. Millipore uses a licensed tool to review customers' parameters and look at how resources are affected by use of the Mobius Solutions, in terms of cost and consumption of resources. Significant savings in water, materials and labour have been found in some processes. A 2008 study [4] of the environmental footprint of a stainless steel-based bioprocessing facility compared to single-use technologies found that single-use systems required:
• 87% less water
• 21% less labour, primarily by reducing CIP activities
• 38% less space
• 29% less energy to run
Future looks towards more automated process
Millipore is helping customers respond to the desire among biopharmaceutical manufacturers to reduce drug development time so they can guard against cash burn while containing their manufacturing costs.
The ultimate goal is to enable small-volume commercial-scale manufacturing of monoclonal antibodies from the bioreactor to final fill/finish using single-use technology options that can be operated in a grey space environment. This longer term vision includes the ability to process products more continuously and with more automation accurately monitoring the process from beginning to end. Next generation products are already looking at how to make processing less manual.
References
1 Leerink Swann, Life Science Tools, Isaak Ro and Jing Dai, March 25, 2009; Evaluate Pharma, Analyst Consensus Data, April 2009.
2 Purifying Therapeutic Monoclonal Antibodies, Amit Mehta, et al, Chemical Engineering Progress, SBE Special Section: Bioprocessing, May 2008, Vol. 104, No. 5.
3 RFID as a Tool for Pharmaceutical Process Conformity and Monitoring, internal Millipore document, Aaron Burke, August 2009 (draft).
4 A Sinclair, L Leveen, M Monge, J Lim and S Cox, The Environmental Impact of Disposable Technologies, BioPharm International (2008).





