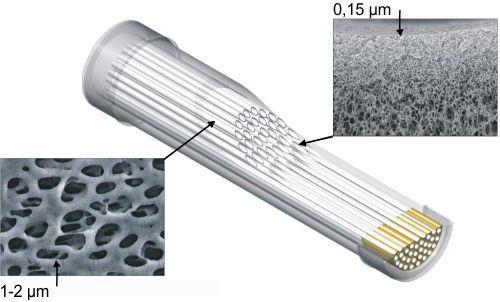
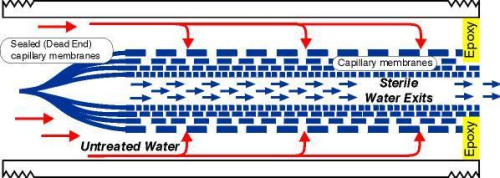
The standard test method for determining bacterial retention in membrane filters requires a one-time challenge of 107 bacteria per cm² of the effective membrane surface [ASTM F838-05 Standard Test Method for Determining Bacterial Retention of Membrane Filters Utilized for Liquid Filtration]. In fact this method replicates what normally occurs gradually in a sterilising filtration process. The method used originated from the point of view that the bacterial load of a membrane filter occurs over time and over the service life of the filter. The goal was to determine if the filter retains a total bacterial load of 107 per cm² (1010 bacteria/ filter cartridge) over its anticipated service life of approximately 5000 L. ASTM F838-05 prescribes Brevundimonas diminuta as the test organism. However, as there is no specific selective culture medium for this organism, it is not possible to differentiate Brevundimonas diminuta from other bacteria. Therefore the tests were carried out with a substitute, the Pseudomonas aeruginosa bacterium for which specific culture media exists. Another reason that Pseudomonas aeruginosa was used is that it has a higher passage percentage than Legionella pneumophila [Influence of size, shape and flexibility on bacterial passage through micro pore membrane filters. T.Egli – Environmental Science & Technology, 2008, 42, 6749-6754.], which is the target bacteria strain to be retained. The choice to perform the validation with Pseudomonas aeruginosa is well founded: the smallest width of Pseudomonas aeruginosa bacteria is 0.50 μm, whereas the smallest width of Legionella pneumophila is 0.55 μm (versus the smallest width of Brevundimonas diminuta of 0.40 μm). For the dynamic test method two cubitainers were filled with tap water filtered over carbon and micro-filtration cartridges in order to ensure sterility of the test water. The containers in which bacterial suspensions were stored were placed in parallel. The bacterial suspensions were consecutively pumped through 2 Legionella Safe shower filters (2-3 bar). Filtration was achieved by means of a super-efficient hollow fibre micro-filtration membrane, with a maximumum pore size of 0.15 μm, which has a high affinity for water by virtue of a unique blend of polymeric components. It only needs low pressure to start the filtration process and delivers high flow rates at a low pressure gradient. The membrane filters (manufactured by Prime Water bvba) consist of a bundle of asymmetric hollow fibre membranes in a tube, where the space between the membranes and the tube is hermetically sealed with an epoxy resin (see Figure 1). In the so called ‘Dead end’ filtration technique (see Figure 2), the water flows from the outside to the inside and the filtered particles are caught in the porous membrane wall, which gradually clogs. A decrease in flow rate to an unacceptable level is a signal for replacement of the filter. This micro filtration technique is primarily applied in Legionella Safe shower filters. From both containers the inlet bacterial suspension was continuously sampled to determine the exact inlet concentration of Pseudomonas aeruginosa. With intervals of approximately 1000 L permeate samples were taken from each of the filters. The flow rate at the end of the test was reduced to approximately 5 LpM, from 10 LpM at the start. After a simulated service life of 5000 and 4500 L respectively with water gradually contaminated with up to E+10 CFU Pseudomonas aerigunosa bacteria, the bacterial retention of both filters was calculated by the analysis of permeate samples at the beginning and the end of two days of filtration. It was concluded that both filter cartridges effectively stop bacteria in the inflowing water and demonstrate a log reduction of at least 6 (99.9999%). The ASTM F838-05 criteria were satisfied in the determination of the bacterial retention of membrane filters.




