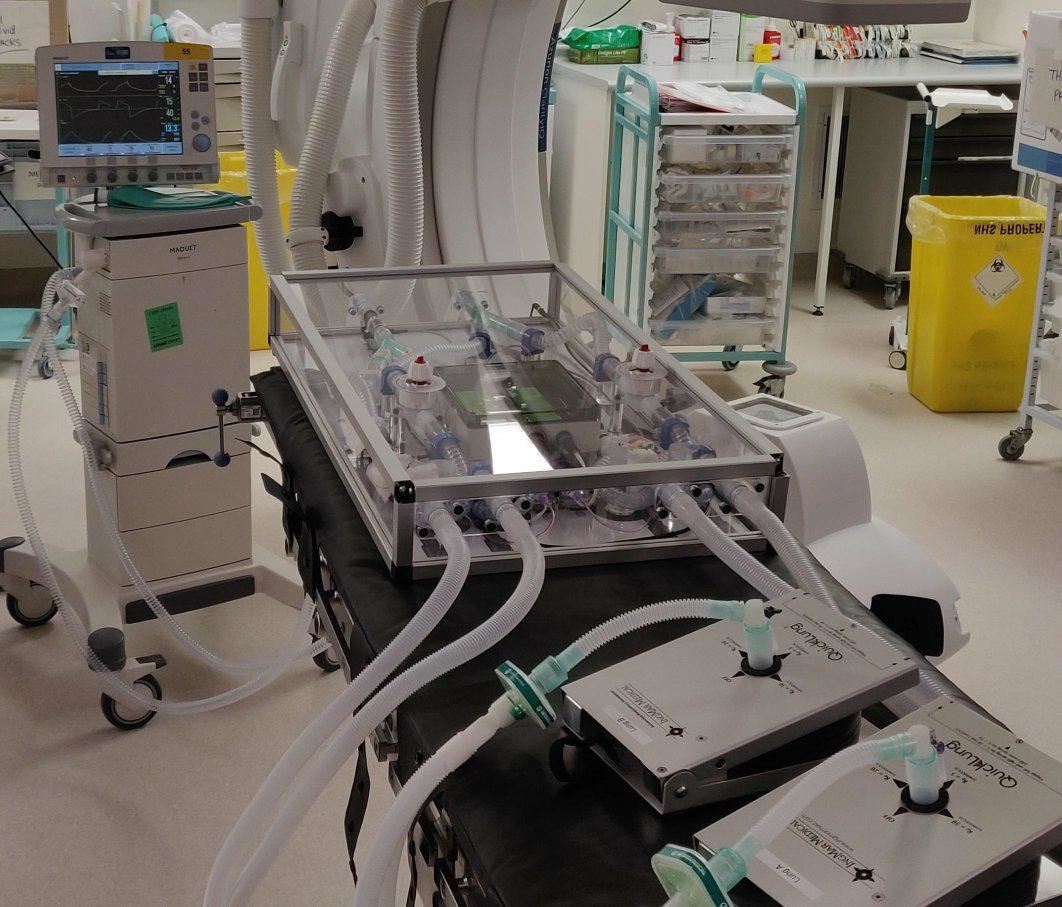
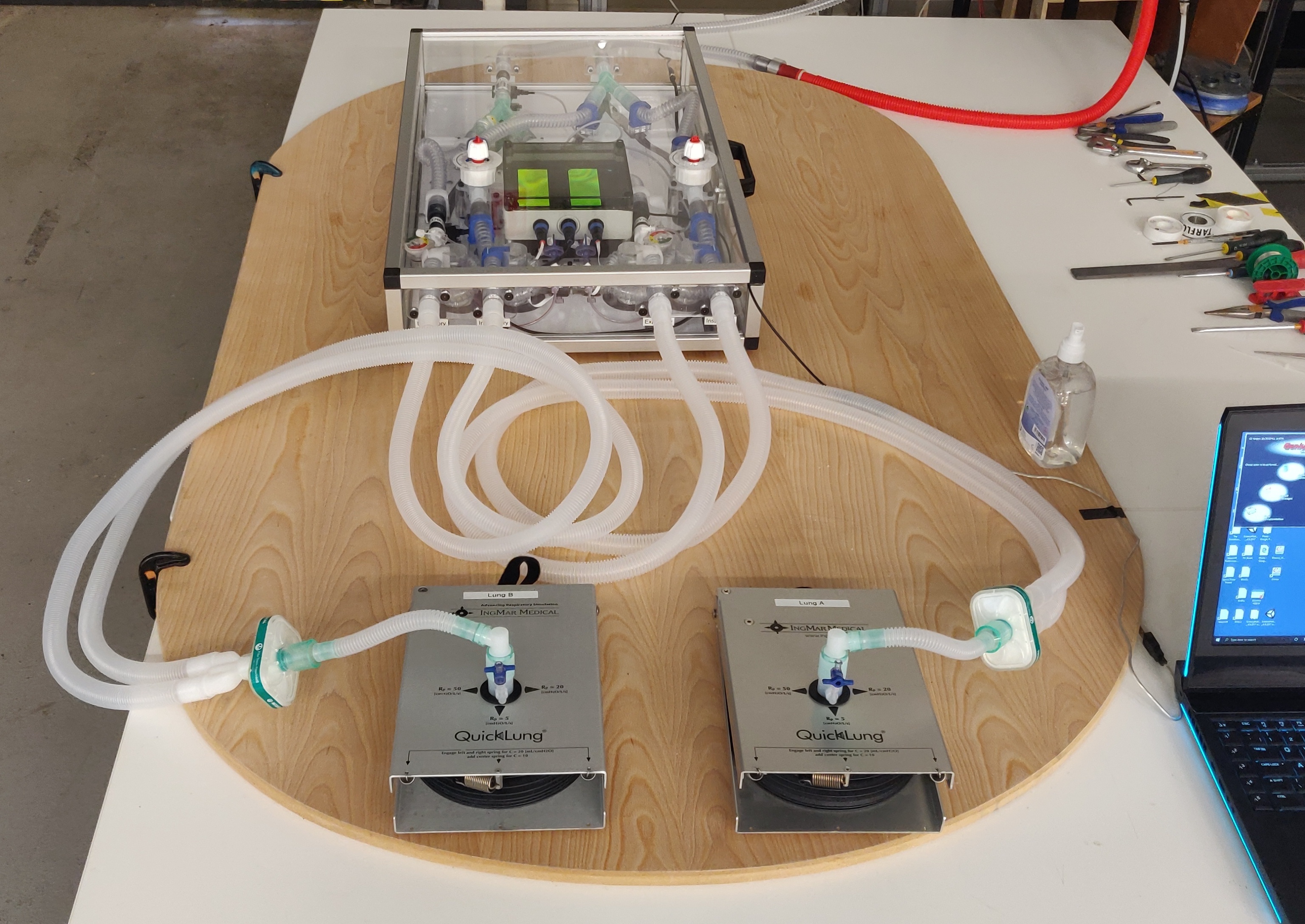
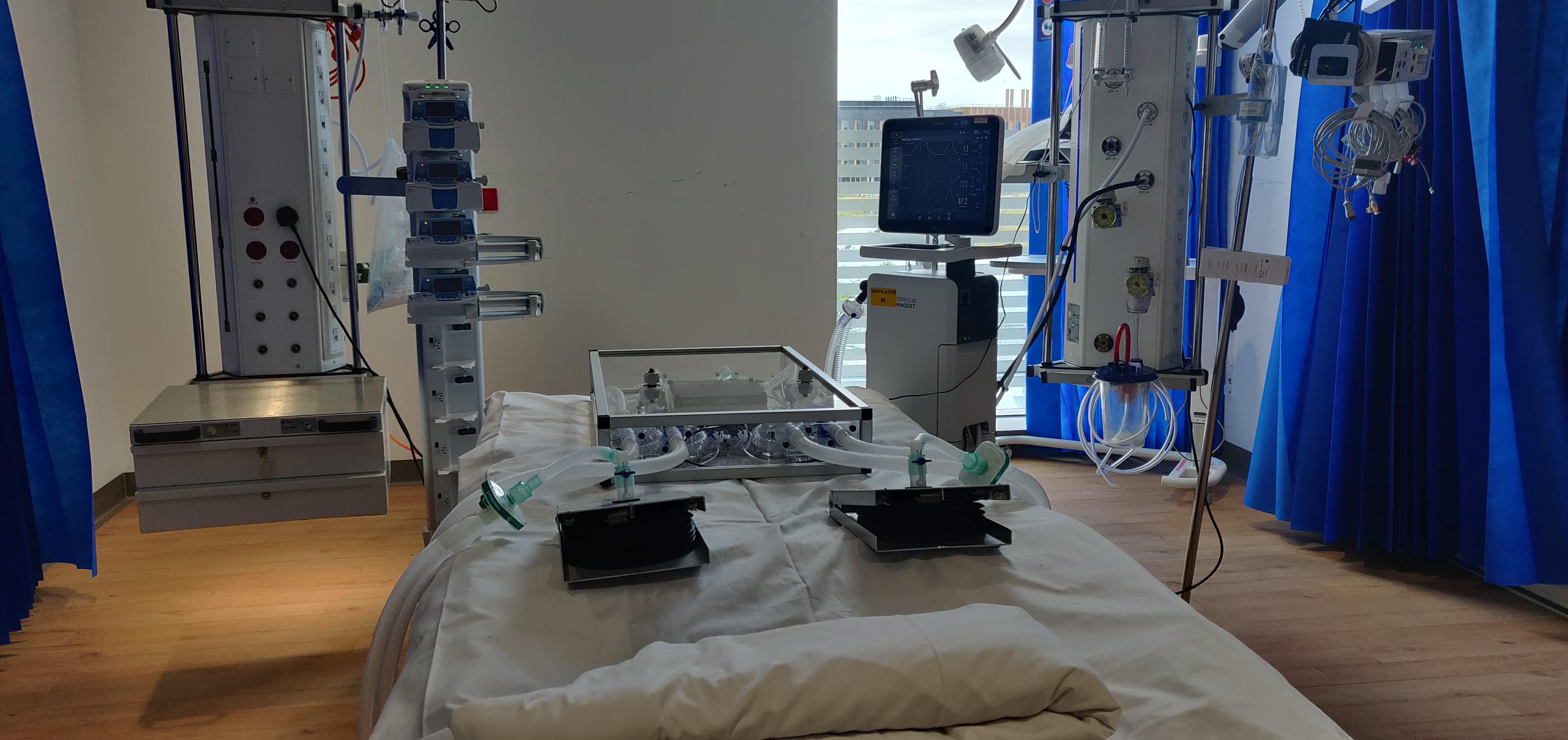
A new ventilator sharing device for Covid-19 patients is being tested by Institute for Manufacturing engineers and anaesthetists from the Royal Papworth Hospital. The device makes it possible to split the airflow from one ventilator, allowing two patients to receive tailored respiratory support.
In response to the Covid-19 pandemic, volunteers from the Institute for Manufacturing (IfM) at the University of Cambridge responded to a request from clinicians at the Royal Papworth Hospital to develop a device that, if needed in an emergency, could be attached to a ventilator to mechanically support the breathing of two sedated Covid-19 patients with different lung capacities and changing breathing needs.
The success of the UK government’s Ventilator Challenge meant that the Royal Papworth Hospital NHS Foundation Trust, along with other NHS hospitals, received enough ventilators to meet the surge in demand from Covid-19 patients and continue to have a healthy reserve stock of ventilators.
However, although ventilator demand in the UK has reduced, the new device is still undergoing testing and, although not yet available for clinical use, it could be adapted to provide emergency support in the future to hospitals in other countries still facing significant challenges with the pandemic, or for longer-term use in countries that have ongoing ventilator capacity shortages.
Doubling capacity
The specification of the active ventilator sharing device was detailed by two anaesthetists, Professor Andrew Klein and Dr Chinmay Patvardhan, from the Royal Papworth Hospital who initially feared that they may not have enough ventilators for patients during the pandemic. When they searched for alternative emergency solutions, they were not confident in the ventilator splitter setups they saw described online.
Professor Klein said: “In case there were insufficient effective ventilators available due to the Covid-19 outbreak, we wanted to have the option, in an emergency, to split and isolate the air delivery from one ventilator between two patients.
"Therefore, we also needed to have the ability to measure and control the air flow to each patient individually and have confidence that if there was a decline or improvement in breathing in one patient this would have no effect on air delivery to, or monitoring of, the other. It was also essential that the setup was easy to assemble.
"The first tests of the device using artificial lungs are very encouraging; the solution developed both meets and exceeds our expectations of many of the other options developed elsewhere around the world. This device could allow medical professionals to instantly double the capacity to ventilate patients safely in a crisis situation which would be invaluable for any future emergency of the scale we have seen with Covid-19,” Dr Patvardhan added. “It could play an important role in those countries still seeing a rise in infections or be used in places where the capacity of the healthcare system is particularly strained.”
Collaborative effort
The IfM developed the solution in collaboration with Cambridge Design Partnership (CDP) as well as the Royal Papworth Hospital, with generous support from a wide selection of respiratory equipment manufacturers across the UK and the world.
The system was designed to incorporate respiratory parts that are available in the UK supply chain in a layout that facilitates parts to be easily changed and replaced. The overall box unit provides isolated respiratory lines to two patients and enables quick assembly to a ventilator.
The IfM-designed readout meter delivers real-time measurement of tidal volume to each patient as well as monitoring the total pressure and airflow in the device. Easy-to-access valves enable fine tuning of air flow to match the requirements of each patient.
The system is currently undergoing a series of tests devised in response to the MHRA emergency guidelines. These use a range of ventilators and tuneable artificial lungs that can simulate patients with different levels of lung compliance. Tests also examine the interaction of the standard ventilator alarm systems with the sharing device, to help understand the changes needed to ensure medical staff are notified of any degradation in patient health or sudden adverse events. A full report of tests results will be released online when they are completed.
Specifications online
Initial details of the specification of the system are now freely available on the IfM website. The site will be updated with all the findings, full design and software when the tests are released so that if other communities across the world have to consider this emergency option in the event of a shortage of ventilators, they can copy and adapt the setup for their own needs.
Dr Ronan Daly, who led the IfM team, said: “We have received incredible support for this initiative across the University and beyond. The team of engineers are making great progress with the device, from the detailed design and fabrication work to preparing regulatory documents, but a wider expertise from colleagues in finance, buildings management, purchasing and safety meant we responded at incredible speed. This project is a small example of a wider national attitude and response of which we should be proud.”
Jon Cooke, who co-ordinated the regulatory and design support received from Cambridge Design Partnership, said: “It was great to be able to support the IfM team and work with them and Royal Papworth to deliver a functioning design in such a short space of time. The combination of the deep regulatory knowledge and robust design techniques of the CDP team along with the great conceptual and experimental work conducted by the IfM made for a truly rapid development process. I’m extremely proud of what the team achieved in a short space of time. While we hope it will never have to be used, we truly believe that this could have a positive impact across the world.”
This article was supplied and written by Jason Naselli, Content and Communications Manager at the Institute for Manufacturing, University of Cambridge.






