Desalination by aromatic polyamide (PA) composite membranes is currently a key technology for solving the global freshwater crisis. However, understanding the main factors influencing water diffusion in the desalination process remains a major hurdle in designing new membrane materials.
Here Chinese researchers investigate the key roles of the hydrates, the nanochannels in a PA membrane, and the functional groups of the membrane on water diffusion by molecular dynamic simulations.
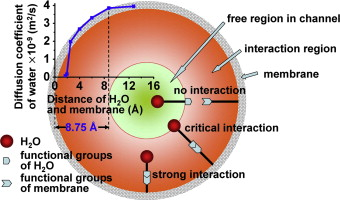
The results show that the membrane can strongly hinder the diffusion of water molecules via both geometrical obstruction and water–membrane interactions when the radius of the water diffusion channel in the membrane is <8.75 Å.
In a dilute NaCl solution, the effect of salt ions on the overall diffusion behaviour of water can be neglected, because only a small quantity of water molecules form hydrates.
On the other hand, almost all Na+ and Cl− can form hydrates, which could explain why the PA membrane has a higher rejection of salt ions than water in seawater.
Desalination, Volume 333, Issue 1, 15 January 2014, Pages 52–58.
