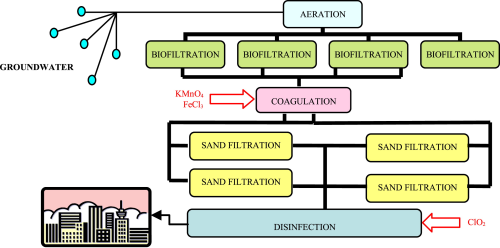
Disinfection is the last treatment stage of a drinking water treatment plant (DWTP), and is carried out to maintain a residual concentration of disinfectant in the water distribution system.
Chlorine dioxide (ClO2) is a widely used chemical employed for this purpose. The aim of this Italian work was to evaluate the influence of several treatments on chlorine dioxide consumption, and on chlorite and chlorate formation in the final oxidation/disinfection stage.
A number of tests were performed at laboratory scale, employing water samples collected from the DWTP in Cremona, Italy.
The following processes were studied: oxidation with potassium permanganate, chlorine dioxide and sodium hypochlorite, coagulation/flocculation with ferric chloride and aluminum sulfate, filtration and adsorption onto activated carbon.
The results show that the chlorine dioxide demand is high if sodium hypochlorite or potassium permanganate are employed in pre-oxidation. On the other hand, chlorine dioxide leads to the highest production of chlorite and chlorate.
The coagulation/flocculation process after pre-oxidation shows that chlorine dioxide demand decreases if potassium permanganate is employed as an oxidant, both with ferric chloride and aluminum sulfate.
Therefore, the combination of these processes leads to lower production of chlorite and chlorate.
Aluminum sulfate is preferable in terms of the chlorine dioxide demand reduction and minimisation of the chlorite and chlorate formation. Activated carbon is the most effective solution, as it reduced the chlorine dioxide consumption by about 50% and the disinfection by-product (DBP) formation by about 20–40%.





