

Pervaporation and vapour permeation have been established over the past two decades as an improved technology for the dehydration of organic solvents, such as alcohols, ethers, esters and ketones, as well as for the removal of organics from aqueous streams or separating various types mixtures of organic compounds. The optimal use can be achieved if this technology gets part of a hybrid system, for example, in combination with distillation and rectification columns.
Introduction
Pervaporation and vapour permeation are membrane-based processes for separating binary or multi-component mixtures of miscellaneous organic fluids. The separation of the mixtures is effected by means of a membrane - the pervaporation membrane. These non-porous (‘dense’) pervaporation membranes made of polymeric or ceramic materials exhibit different permeabilities towards different components, resulting in the desired separation of the components. In the process, the feed is first heated up to the operating temperature and then brought into contact with the active (feed) side of the pervaporation membrane. The better permeating component preferentially passes through the membrane and is continuously removed in the form of vapour from the back (permeate) side of the membrane. The continuous removal of the vaporous permeate creates a concentration gradient across both sides of the pervaporation membrane. This concentration gradient acts as a driving force for the process.
Basic principles
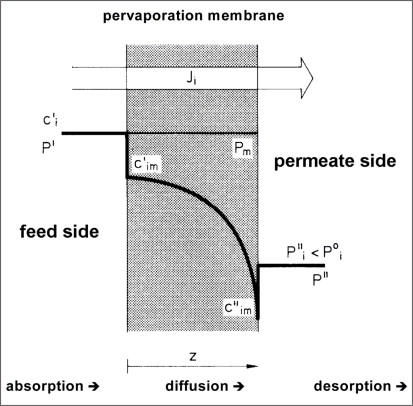
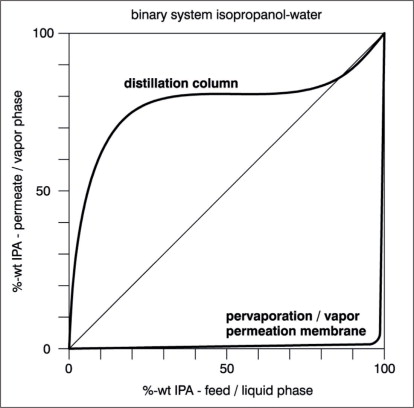
The concentration gradient is best expressed in terms of partial vapour pressure. A number of different models have been developed to describe the pervaporation process, but for the sake of simplification, the mass transfer across a pervaporation membrane can be divided into three major steps: • Sorption of permeating components at the • feed side into the membrane. • Transport of absorbed components across the • membrane by diffusion according to Fick´s law. • Desorption at the permeate side into • vapour phase under vacuum. Figure 1 illustrates these three steps (c = concentration/p = partial vapor pressure/J = mass transfer rate of permeating components). Two values characterise a membrane: • Its selectivity (also called separation0 characteristic); and • The permeate flux (or mass transfer rate) across the membrane. A slightly modified McCabe-Thiele diagram can be used to demonstrate the selectivity of a pervaporation membrane in comparison to the vapour-liquid-equilibrium. Such a diagram is shown in Figure 2 for the binary system isopropanol-water. The composition of the permeate is plotted over the composition of the feed. Included in the diagram is the composition of the vapour, in equilibrium with the liquid mixture. In contrast to this equilibrium curve, water is the more permeable component at nearly all feed concentrations. Depending on the type of pervaporation membrane, up to about 95-97 wt.% of isopropanol, the composition of the permeate is constant and independent from the feed composition.
Pervaporation process
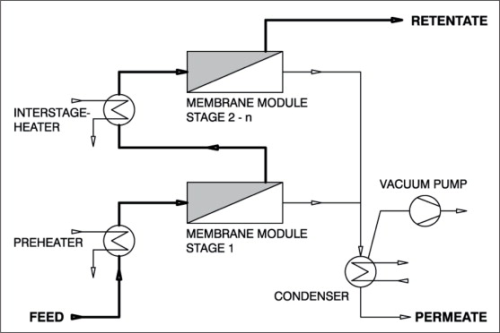
In the pervaporation process, as shown in Figure 3, a liquid feed stream is first pre-heated to operating temperature and then routed to a membrane module. The permeate transported through the membrane is vaporised on the permeate side of the membrane and heat is dissipated from the feed. As the partial pressure of the transported component, and with it the driving force for mass transportation, decreases at declining temperature, the feed mixture has to be re-heated. In most cases, re-heating takes place outside the modules in separate heat exchangers. Therefore for larger plants and high permeate rates, it may be necessary to provide for a very large number of small membrane modules with upstream heat exchangers. The vaporous permeate leaving the membrane module is condensed in an external heat exchanger. The vacuum pump is only used for the removal of the inert gases, but has no other function in the process.
Vapour permeation process
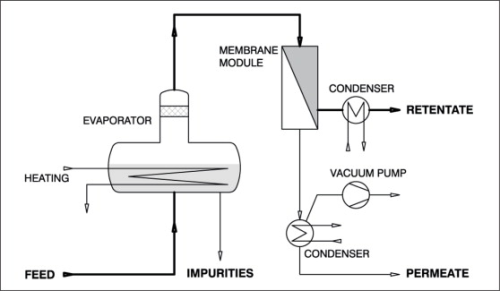
In the vapour permeation process, illustrated in Figure 4, saturated vapour instead of the liquid feed solution is passed through the module. This process - similar to gas separation - exhibits some other advantages over pervaporation. The series arrangement of modules and heat exchangers can be dispensed with because the necessary evaporation energy is supplied from outside the modules in a separate evaporator. Because of the more favourable fluid dynamics, overall larger modules may be used with an associated benefit of cost reduction. Moreover, vapour permeation is advantageous if the feed mixture contains non-volatile or undissolved constituents and any of its constituents that tend to precipitate out can be separated as bottom product in the evaporator.
Main advantages
The main advantages of a pervaporation or vapour permeation process may be summarised as follows: • Since only the properties of the membrane • determine the distribution of a component in the permeate phase, mixtures which at normal distillation form azeotropes and/or require a large number of theoretical stages (like the dehydration of acetone), can easily and economically be separated even without the use of entrainers. Therefore, high product purity is obtained (no entrainer required) and no environmental pollution occurs (no entrainer emitted). • Multi component mixtures even with just small differences in boiling points can be dehydrated effectively and economically. • The feed mixtures to be treated may be supplied in either liquid (→ pervaporation) or vapour (→ vapour permeation) form. • Low energy consumption for pervaporation and vapour permeation processes. • Significantly reduced energy consumption for hybrid systems (pervaporation and vapour permeation in combination with rectification/distillation). • Due to the modular design of the membrane system even small units can operate economically. • High degrees of flexibility regarding the feed mixtures that may be accommodated (multi-purpose systems, various feed mixtures can be treated in one unit), throughputs, and final product qualities. • Modularly, compactly designed, and factory-preassembled systems simplify their adaptation to suit the desired performance parameters and shorten the time required for system installation and start-up. • Pervaporation and vapour permeation systems are simple to operate and can be started up and shut down rapidly.
Feed materials
The following summary presents an overview of some important substances that can be treated by pervaporation or vapour permeation: • Alcohols such as methanol, ethanol, propanols, butanols and higher linear alcohols, as well as higher alcohols such as glycol, glycerin and glycol ether on consultation. • Ketones such as acetone, methyl ethyl ketone (MEK) and methyl isobutyl ketone (MIBK). • Ethers such as diethyl ether, diisopropyl ether, tetrahydrofurane (THF) and dioxan. • Esters such as ethyl acetate, butyl acetate and isopropyl propionate. • Hydrocarbons such as benzene, toluene and xylene, (in most cases in mixtures with other solvents), as well as chlorinated hydrocarbons such as trichlorethylene. • Organic acids such as acetic acid, propionic acid and aqueous solvents higher acids. • Amines (on consultation) such as methylamine, ethylamine and pyridine. Aprotic solvents like DMF, DMSO or NMP will attack polymeric pervaporation membranes, but can be dehydrated by means of ceramic pervaporation membranes.
Major applications
Some typical applications for pervaporation and vapour permeation include: • Removal of water from organics. • Removal of organics from water. • Separation of organic mixtures. • Concentration of aqueous solutions. With respect to the number of installations, installed membrane area and economical advantages, the removal of water from organic solvents and solvent mixtures is the most important pervaporation and vapour permeation process. Membranes, modules and process know-how are fairly well developed and allow the installation and operation of industrial plants with large capacities. Removal of organics from water is a minority application; demonstration and industrial plants are in operation, but the process has still to prove its economic viability in larger capacities. This is particularly the case for the treatment of wastewater and gases such as air, where relatively often very low final concentrations have to be removed. Separation of organics by pervaporation/vapour permeation is still a growing sector. Membranes have been developed and are reportedly tested on a pilot plant and industrial scale, for example, in the recovery of methanol in the production of MTBE or for the reduction of benzene concentrations in gasoline or for separation of aliphatics from aromatic solvents.
Solvent dehydration processes
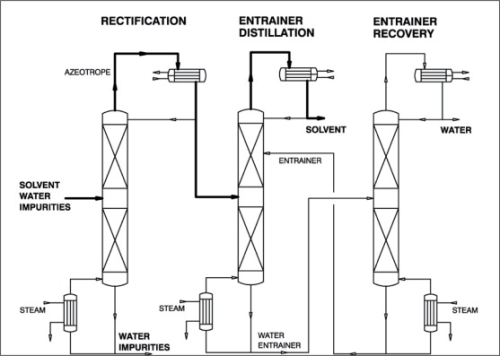
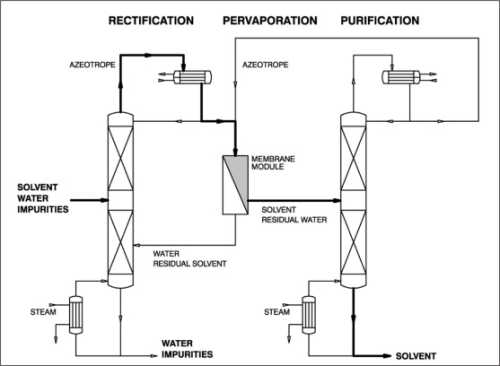
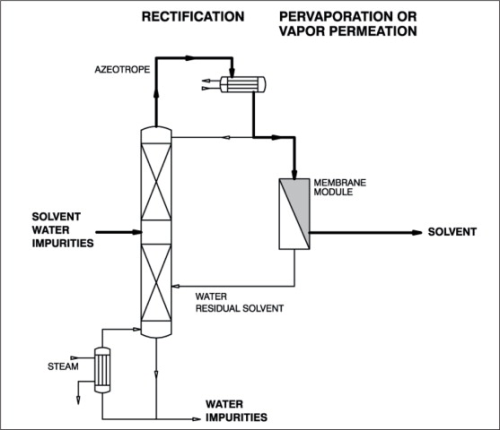
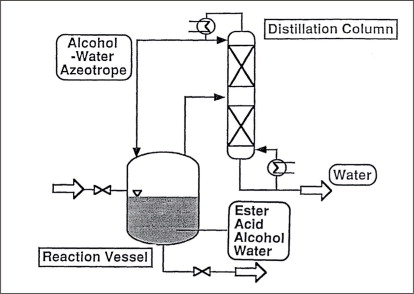
When simple distillation is practical, pervaporation or vapour permeation is usually not economical. However, where distillation requires entrainers to split azeotropes and/or a large number of theoretical separation stages and/or high reflux ratios, the employment of pervaporation or vapour permeation must be taken into consideration. Figure 5 illustrates a typical process with pre-distillation, extractive distillation and entrainer recovery for the purification and dehydration of polluted solvents, for example, for a system isopropanol-water system. How such a standard process can be improved by integration of a pervaporation or vapour permeation unit is shown in Figure 6. A pervaporation or vapour permeation process will show the greatest advantages in hybrid systems for the separation of those mixtures in which the organic component has been pre-concentrated by distillation to a certain degree. The optimum pre-distillation depends on the nature of the organic component, but is often a sub-azeotropic concentration. Furthermore, during pre-distillation the untreated raw feed mixture is also pre-purified by removing all non-volatile impurities. As with all membrane processes the feed stream to a pervaporation membrane should be free of undissolved particles and should not contain dissolved substances, which could precipitate out during the concentration process. Such particles otherwise could block or even destroy the membrane. The pre-distilled product (azeotropic concentration) is drawn from the head of the first column and then fed to the pervaporation unit. During the pervaporation process, the feed mixture will be dehydrated above the azeotropic point and then passed to a second column for final dehydration/purification purposes. The water-rich, vaporous permeate should be condensed and recirculated to the pre-distillation column in order to minimise losses of the organic solvent if necessary. This hybrid process is also applicable to systems such as tetrahydrofurane-water and pyridine-water for example. Depending on the nature of the solvent, overall capacity and product quality, it might be more economical to remove the second dehydration/purification column and apply the pervaporation unit for final dehydration as well. This process variation is illustrated in Figure 7. One successful use of pervaporation and vapour permeation is in dehydrating esterification mixtures, in order to increase reaction efficiency and yield. Figure 8 and 9 demonstrate the possibility to enhance the esterification process by integration of a pervaporation unit. The alcohol is used in excess and an alcohol-water mixture is evaporated out of the reaction vessel and passed to a distillation column. Water is separated from the bottom of the column also by means of a pervaporation unit.
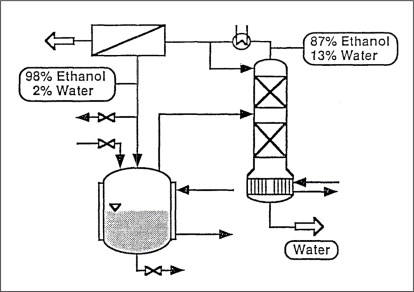
Case study 1: Chemicals
A batch operated process in the chemical industry generates several tonnes of a binary isopropanol-water mixture contaminated with dissolved solids every day. The water concentration of around 11-12% is very close to the azeotropic composition. The objective was to remove water to less than 0.5% and recycle pure alcohol without any traces of other chemicals back to the process. An external treatment was rejected because of logistical reasons and overall costs for handling and treatment. Dehydrating such alcohols by distillation/rectification requires a complex and space consuming process unit as well as an entrainer, leading to impurities in the dehydrated solvent. It was decided to install a turnkey, customer-designed and skid-mounted vapour permeation unit. The plant operates continuously and separately from the batch process. Such membrane systems are compact, for example, they can easily been incorporated in any existing infrastructure onsite and are simple to operate (once-through process). The membranes separating azeotropic mixtures do not require any additives such as entrainers or other chemicals.
Case study 2: Pharmaceuticals
The aim was to recover pure solvents (a purity of at least 99%) from a ternary mixture of ethanol (approx. 70-80%), xylene (approx. 2-15%) and water (approx. 15-20%) contaminated with dissolved and undissolved solids as well as traces up to several 100 ppm of high boilers, fats, and several organic chemicals/pharmaceuticals. Ethanol and xylene both build azeotropes with water and therefore cannot be separated or dehydrated by any simple distillation. Dehydration of the ternary azeotropic mixture by pervaporation technology now permits economic separation of both solvents. A three-stage hybrid system distillation-pervaporation-distillation was installed to purify, dehydrate and separate both solvents up to greater than 99%. At the start a simple distillation column generates a purified ternary ethanol-xylene-water mixture. Following this a pervaporation system removes the water from the purified ternary mixture. This membrane process now allows an easy separation of the remaining binary ethanol-xylene mixture in a simple distillation column. The solvent recovery plant was designed as a multi-purpose system and is also used to treat an isopropanol-xylene-water mixture (composition as before). The complete system has proven its reliability with continuous operation over the past ten years.
Case study 3: Solvent recycling and organic/organic separation
A company recovering solvents from different industrial sources required a technology to dehydrate volatile organic solvents such as alcohols, esters, ethers and ketones. The majority of these solvents built azeotropes with water and/or required a large number of theoretical stages for dehydration by distillation/rectification, like acetone. Due to the fact that feed volume as well as solvent composition and water concentration of the incoming raw solvent mixtures vary significantly, a flexible, multi-purpose and easy-to-operate system was requested. A simple stand-alone batch pervaporation plant is able to dehydrate binary and multi-component solvent mixtures with 2-20% water down to any desired final water concentration. A batch tank is used and a feed stream is circulated from the batch tank through the membrane unit and back to the tank until the final water concentration is reached, typically 0.1-1.0%. The membrane area is arranged in several stages with relevant feed re-heaters to balance the temperature drop while handling solvents with high water concentrations. Like water, methanol also forms azeotropes with a lot of commonly used solvents. The pervaporation system is also able to remove methanol instead of water from these solvent mixtures.





