



The presence of iron in ground water is a direct result of its natural existence in underground rock formations and precipitation water that infiltrates through these formations. As the water moves through the rocks some of the iron dissolves and accumulates in aquifers which serve as a source for ground water.Since the earth's underground rock formations contain about 5% iron it is common to find iron in many geographical areas around the globe.Iron in water is typically found in three major forms and is rarely found in concentrations greater than 10 milligrams per litre or parts per million (mg/l or ppm):• ‘Clear water iron’ is a non-visible ferrous (Fe2+) form of dissolved iron is found in water that is not exposed to oxygen, such as in wells and springs.• ‘Iron bacteria’ – Dissolved iron contributes greatly to the growth of iron bacteria. These bacteria form dark-coloured slime layers on the inner walls of the system's pipes.
The problem
In surface water, such as rivers and lakes, dissolved iron is hardly ever found, because it reacts with oxygen, forms insoluble compounds and sinks out of the water. However, in ground water such as wells and springs, iron is the most common dissolved chemical. Although not considered to cause health problems in humans, its presence in potable water is rather unpleasant due to the bad odours it spreads, its rusty taste and colour, its feel on skin and hair, and its tendency to stain clothing.In addition, the presence of dissolved iron enhances the growth of iron bacteria, which forms dark-coloured slime layers on the inner side of a system's pipes. The slime is then released into the network through water flow fluctuations, leading to dirt build-up and damage to the plumbing.The problem of dissolved iron is highlighted in Figure 1 which was taken in the city of Ramenskoe (a Moscow suburb), where local wells containing 0.8-4 mg/l of iron serve as the city's potable water source. In fact, the discomfort in this city is so severe that bottles of mineral water are provided to hotel guests for washing.Most countries have accepted a safe drinking water standard (aesthetic, not health related) with a maximum of 0.3 ppm iron content. Water drawn from sources with higher iron content should be treated before entering any municipal water supply system.At the same time, iron is an essential nutrient for humans, with a recommended daily intake of 5 milligrams. Therefore, the official water and environment agencies in many countries have established a secondary limit for iron in drinking water, which is based on aesthetic concerns (Secondary Maximum Contaminant Level – SMCL).In countries such as the US, Canada, Greece, Iran, Russia and others, the SMCL for iron in drinking water is 0.30 mg/L (milligrams per litre) or ppm (parts per million).
Primary solutions
In general, there are two well-known iron removal technologies from which to choose. However, it should be noted that these technologies are not applicable to all local conditions and managers need to evaluate each of them for their specific needs:• Bypassing the problem• Removing the iron concentration in cases of levels higher than 0.3 ppm
Bypassing the problem
Sometimes simply finding an alternative water source may prove to be the most cost-effective solution• In cases where alternative iron-free water sources are found nearby, the cost of installing transmission lines needs to be compared against the cost of removing the iron from the local water.• In cases of wells with high levels of iron, it may be considered possible to drill into different aquifers instead of using the contaminated wells in order to bypass the problem. It may also be possible to mix water from different sources in order to meet the iron level standard.Unfortunately, in cases where iron has contaminated large aquifers, or when alternative water sources are too far away, bypassing the problem is not possible.
Removing iron by oxidation and filtration
In cases of iron levels higher than 0.3 ppm, sequestration is not possible, and therefore the iron must be physically removed from the water.Removing dissolved iron is a two-stage process:• The first stage is oxidising the dissolved iron and transforming it from soluble form to insoluble ferric form, where the small oxidised iron particles (rust) become suspended in the water.• In the second stage, it is necessary to remove the suspended particles from the water. This process is regularly done through filtration, and its success is entirely dependent on the quality of the filtration process. Improper and insufficient filtration may jeopardise the entire iron removal process.
Iron oxidationThere are many methods for oxidising iron, including softening it with lime or by using agents such as chlorine dioxide (ClO2), ozone (O3) or by potassium permanganate (KMnO4). However, the most cost-effective, environmentally friendly and commonly used method for oxidising iron is by aeration.Iron is easily oxidised by atmospheric oxygen and the aeration process provides the dissolved oxygen needed to convert the iron into an insoluble form, without the use of chemicals. It takes 0.14 ppm of dissolved oxygen to oxidise 1 ppm of iron.It should be noted that the aeration process requires careful control, as insufficient air flow will not properly oxidise the iron. At the same time, if the air flow is too high, the water may become saturated with dissolved oxygen and become corrosive.Two methods of aeration are commonly used: dispersing the water into the air and bubbling air into the water. Other aeration methods include the use of cascade trays, cone aerators, and porous air stones.It is important to ensure successful completion of the aeration process by allowing at least 20 minutes of detention time before filtration. This is regularly done by installing a reaction tank downstream of the aeration basin. The water pH affects the reaction time and therefore it is necessary to monitor and correct the pH during the detention process.
Common filtration technologiesAfter the oxidation stage, the precipitated material (Fe(OH)3) should be removed from the water, either by filtration or by sedimentation followed by filtration.There are many filtration technologies that may be used for removing oxidised iron from water, including steel strainers, layered screen filters, disk filters, deep media filters, textile fibres and cartridge filters.A common filtration technology is deep media filtration, in which the filters remove the oxidised iron particles through a thick layer of graded sand, gravel or other granular materials. With this method, the filtration rate depends on the active surface size of the bedding, as well as the velocity at which the water flows through the filter.However, the media filtration method has some major drawbacks, including a very high percentage of rejected water. In addition, iron bacteria cause the media particles to stick together and allow unfiltered water to flow freely through gaps created in the filter media. Ultimately, the filtration quality drops over time and the unclean bedding causes bacteria and other unwanted organisms to thrive.
Micro fibre filtration for iron removalSelf cleaning micro fibre filtration is a new technology combining the filtration effectiveness of cartridge filtration with the low operational costs associated with self-cleaning filtration technologies. It has proven to be very effective in removing oxidised iron particles generated by a regular oxidation process of water that was contaminated with dissolved iron.The basic component of this technology is the cassette, which is a filter media consisting of a grooved rigid plastic plate over which multi-layer textile threads have been wound. The thread type and tension, together with the number of layers, defines the filtration degree, which can range from twenty down to two microns.The cassettes are connected to a collector pipe and form a unified package; the packages are attached to one another to form a cartridge, which is then installed in the filter housing.The water containing the oxidised iron flows through the threads, into the grooves and through the collector pipe to the customer's system.The large iron particles are immediately blocked on the surface of the multiple layers of thread, while finer particles that penetrate the surface are trapped deeper inside the thread layers. As the iron is stopped, the filter differential pressure gradually increases.The self cleaning sequence begins when the differential pressure reaches a pre-set level. It shoots high pressure water jets through the thread layers of the cassette, and the jets hit the plastic wall and are forced backwards. This creates a powerful spot back flush, which carries the trapped iron particles out of the cassette's thread layers and to the drain system.One of the significant advantages of self cleaning micro fibre technology over sand media filtration is the ability to overcome the major drawback already mentioned - iron-bacteria growth causing the traditional media particles to stick together and allowing the unfiltered water to flow freely through the gaps and between the lumps. This phenomenon is entirely prevented by using the micro fibre technology, due to the very nature of the cassette's structure.In addition, the percentage of rejected water using the micro fibre filtration technology is much lower than that of the sand media filtration. This greatly reduces the energy and operational costs of the entire iron removal process.
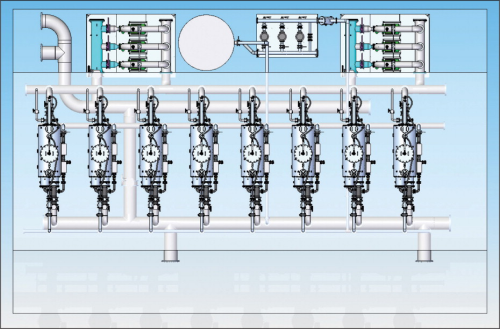
Iron removal system layout
Figure 6 is a typical layout of a micro fibre filtration system operation in filtering oxidised iron particles in an iron removal plant.
Conclusions
The presence of iron in water is a challenge that many operators face. When deciding on how best to remove the iron from your water system, it is important to weigh the pros and cons of each solution presented in this article, from both a feasibility and environmental stand-point, as well as to analyse the short and long term financial impact on the entire system.





