

A pdf version of this article is available on the right-hand side under 'Downloads'
It is not often that the US-EPA registers a novel biocidal preparation, let alone one aimed at the daily disinfection of drinking water. But that is what happened in 2009, when the agency assigned registration # 7208-3 to HaloPure Br, a new medium manufactured for incorporation into devices and systems for treatment of drinking water. Later in 2009 a gravity-feed demonstration product, based on the action of this medium, was also registered (# 7208-4). HaloPure enables the development of untethered water purification devices that do not require electricity or pressure while meeting the rigorous demands of compliance with US-EPA Microbiological Guide Standards. This new medium is already being used in a WQA Gold Seal-certified consumer water purifier product in several markets internationally. Finally, while typically used as a primary disinfectant, HaloPure Br has also demonstrated a reliable capacity to control heterotrophic microbial contamination, and inhibit the formation of biofilm.
Background
What is HaloPure Br, what are its origins, and how does it work to meet these registration requirements safely? Here we provide an introduction to the technology, explore some of its properties, and share the ways they are put to work in an expanding array of applications.
At the outset, it needs to be clarified that the medium is unique in two different ways from most of the water disinfectants that have preceded it:
• Firstly, HaloPure Br is an insoluble polymeric solid that brings about decontamination primarily by contact with microbes in water as it percolates through a bed of HaloPure medium.
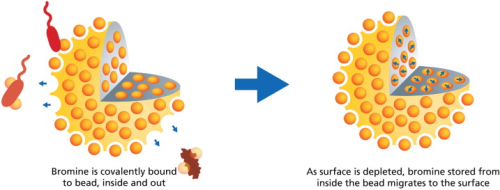
• Secondly, the disinfecting constituent of the particles is bromine (Br), covalently bound to heterocyclic ring structures synthesized de novo (from the beginning) on a substrate of porous, crosslinked polystyrene beads (see Figure 1).
The origins of this chemistry date back to the work of Professor S. D. Worley at Auburn University (Auburn, AL, USA) in the early 1990s. He conceived a new approach to the stabilisation of halogens in which N-halamine bonds would covalently bind in place either chlorine or bromine on the surface of novel polymeric compositions [1].
Many such structures were synthesized, and one, a hydantoinylated polystyrene, was successfully developed at HaloSource Inc. as a commercial product [2].
In the form of uniformly spherical beads (0.3-0.7 mm in diameter), the medium can be integrated into water treatment filtration trains so as to allow for reproducible and attractive flow rates, and predictable antimicrobial capacities.
Choosing bromine
Why bromine? After all, with chlorine having such an established place in the disinfection of potable water worldwide, there would seem little reason to introduce yet another novelty to the ‘solid halogen stabiliser' approach. Bromine has not enjoyed wide use in drinking water disinfection, largely because it offers none of the convenience and versatility of forms that chlorine does. In its elemental form it is a difficult substance to manage; its use has traditionally required significant engineering controls so as to deliver bromine into an aqueous stream at concentrations that meet disinfecting requirements [3]. Yet it has long been clear that as an oxidising disinfectant, bromine shows superior potency and speed over chlorine in its action against both bacteria and viruses [4] and [5], even though it is demonstrably weaker in oxidising power compared to chlorine.
In fact, until this new polymeric medium came along there had never been a convenient way to take advantage of these characteristics of bromine. Furthermore, taking into consideration the additional benefits of working with bromine (i.e. greater tolerance to pH and temperature variations along with much higher taste threshold for residual detection), it fast became the preferred halogen in the HaloPure development process. Once it became apparent that brominated hydantoinylpolystyrene beads could enable water treatment devices to deliver excellent capacities without extensive bead cartridge replacement (see below), HaloPure Br was a good choice for registration.
Bromine's potency against tough waterborne viruses, such as poliovirus [5], and rotaviruses [6] as well as its efficacy against a wide spectrum of bacteria were decisive. Still to come was the need for an extensive exploration of the safety of Br in such an application. This required assurance of compliance in every way including with standards for toxicity of the charged polymer, daily intake of bromine, and the certainty that no untoward by-products were generated either in manufacturing or through interactions with other components of intake water. All such compliances were achieved, and this is reflected in the US-EPA's registration.
Additional key characteristics of this medium emerged in the development process (see Figure 2). Careful control of bromine charging – so that halogenation is confined to a specific site on each hydantoin ring – produces a powerful surface display of bromine atoms for interaction with adjacent microbes. Suitable configuration of the synthetic reaction mixtures allows for hydantoinylation throughout the porous matrix, providing a reserve of bound bromine for migration to the surface as disinfection reactions deplete bromine atoms upon contact at the surface. This accounts for the attractive endurance of HaloPure Br in disinfection/purifying applications. Finally, with appropriate adjustments to the bromination step, a low level, but potent bromine residual can be imparted to the water. This offers, where desirable, a sustained protection of the water that remains below taste and odour thresholds without compromising sensory qualities for the consumer.
Using HaloPure
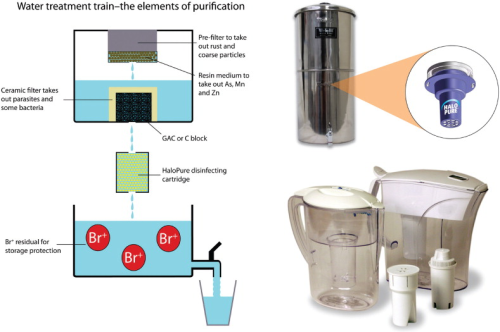
Water treatment trains inevitably involve sequential passage through a series of components. HaloPure Br fits in comfortably with filtration and separation elements conventionally used in point-of-use (POU) water purifiers (particulate removal and absorption/adsorption media, such as non-woven matrices, ceramics, activated carbon blocks and GAC granule beds, ion exchange resins, and potentially specific media aimed at retention of contaminants such as arsenic, or fluoride). Gravity feed devices can be engineered to enable daily disinfection over periods of months, extending the life of the product in a convenient way, to several thousand litres, in some cases. HaloPure Br makes an attractive complement to existing water filtration trains as an ‘add-on' (see Figure 3) or even as a retrofit, supplementing the functionality of popular filtration systems. In this way, potent disinfection becomes a new outcome of water treatment. By adding residual bromine to the product water, as desired, the water can also be protected for storage purposes.
There is good fit between HaloPure Br and POU water filter applications. The proper role for the medium here is to treat potentially contaminated water for disease agents, and lower the risk of exposure to consumers – a ‘primary disinfectant' function.
HaloPure and biofilm
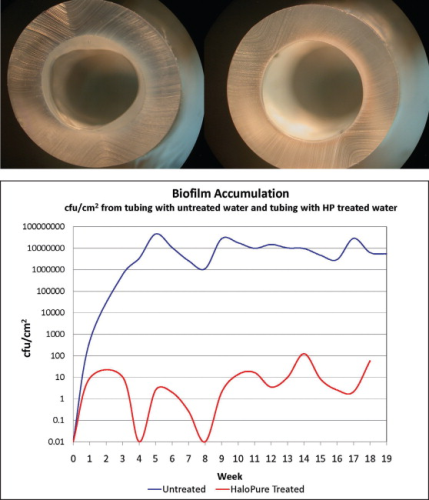
However, the medium is also proving to have unexpected efficacy in limiting the rate of formation of biofilm downstream of the placement of HaloPure Br. This became apparent in simple experiments in which water flowed through polyurethane tubing upstream of which HaloPure Br was installed in the test line, and was absent in the control. The extent of biofilm formation over time was compared. Marked differences were seen after several months of flow (see Figure 4). As a result, longer term experiments were designed to assess potential use of the medium to protect water storage surfaces in reverse osmosis (RO) tanks, and water chiller devices. By comparing the biofilm populations adherent to the surfaces (measured as bacteria per cm2), it was possible to show protracted impacts on colonisation and biofilm slime formation in this kind of application (see Figure 4).
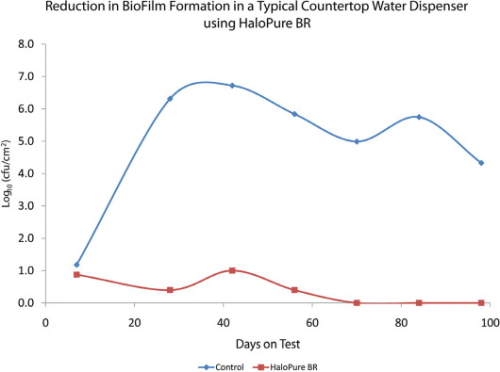
Further investigating this phenomenon, commercially available POU water dispensers that contain a holding tank were set up. A small HaloPure Br cartridge was installed upstream in one of the holding tanks. As Figure 5 shows, biofilm formation on the wall of the tank (expressed as cfu/cm2) was markedly reduced in the HaloPure Br unit versus the control.
There are continuing concerns about the deleterious effects on water quality of biofilm formation in tanks and tubing. Increasing regulatory pressures in many jurisdictions now impose limits on heterotrophic bacterial numbers in product water. Evidently, there is a place for HaloPure Br in this setting. The effectiveness is probably the result of a combination of factors, including a high degree of inactivation of organisms entering the tanks or tubing, combined with the potency of a bromine residual, albeit at low levels (often around 0.2 ppm). But, even allowing for these explanations, the results are unexpectedly good, and lead to suspicions that bromine is responsible for other disruptive events in the normal process of biofilm deposition. This could be valuable as a supplement in preventing slime formation as a cause of impedance in membrane filtration, for example, and is worthy of further exploration.
Overall, HaloPure Br is opening up a wide range of commercial applications. It is bringing value wherever fast, powerful, broad spectrum antimicrobial actions within a water treatment train – optimally in combination with other elements of filtration and separation – can benefit the operations of the devices as well as the consumers of the product water. Already in use in commercial household gravity feed devices in Asia and the Middle East, the scope of its usefulness is expanding.
References
1 S.D. Worley and G. Sun, Biocidal Polymers, Trends in Polymer Science 13 (1996), pp. 301–307 and ‘Polymeric Cyclic N-Halamine Biocidal Compounds’ U.S. Patent # 5,490,986, (1996)..
2 Y. Chen, S.D. Worley, J. Kim, C.-I. Wei, T.Y. Chen, J.I. Santiago and J.F. Williams, Biocidal Polystyrenehydantoin Beads for Disinfection of Water, Industrial Engineering Chemistry Research 42 (2003), pp. 280–284. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (35)
3 http://www.everpure.com/products/Pages/Marine.aspx.
4 D.E. Williams, E.D. Elder and S.D. Worley, Is free Halogen Necessary for Disinfection?, Applied and Environmental Microbiology 54 (1988), pp. 2583–2585. View Record in Scopus | Cited By in Scopus (7)
5 R. Floyd, J.D. Johnson and D.G. Sharp, ‘Inactivation by Bromine of Single Poliovirus Particles.’, Applied and Environmental Microbiology 31 (1976), pp. 298–302.
6 V.S. Panangala, L. Liu, G. Sun, S.D. Worley and A. Mitra, ‘Inactivation of rotavirus by new polymeric water disinfectants.’, Journal of Virological Methods 66 (1997), pp. 263–268. Article | PDF (512 K) | View Record in Scopus | Cited By in Scopus (13)





